Variation over time and interdependence between disease progression and death among patients with glioblastoma on RTOG 0525
- PMID: 25688120
- PMCID: PMC5654351
- DOI: 10.1093/neuonc/nov009
Variation over time and interdependence between disease progression and death among patients with glioblastoma on RTOG 0525
Abstract
Background: We assessed the longitudinal hazard characteristics for death and progression in patients with glioblastoma, evaluated the impact of prognostic factors and treatment on the hazard within different time intervals to determine if effects are time varying, and quantified the influence of progression on survival.
Methods: Among patients randomized to Radiation Therapy Oncology Group trial 0525, which compared dose-dense with standard-dose temozolomide, we estimated the hazards of death and treatment failure (death or progression) over time and their interdependence.
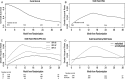
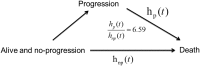
Results: The peak hazard of death was reached at around 16 months with a slow decline after that; the hazard of progression/death reached a peak at around 6 months and decreased dramatically thereafter. The survival advantages for patients with MGMT gene promoter methylation and recursive partitioning analysis class III were substantial in the first 2 years, but lessened thereafter. The progression-free survival benefit of dose-dense over standard-dose temozolomide occurred in the first 6 months (hazard ratio: 0.70; 95% CI: 0.58-0.86; P < .001), although it diminished thereafter. After adjusting for recursive partitioning analysis class and MGMT methylation status, the hazard ratio of death for patients who had progressed over nonprogressors was 6.59 (95% CI: 5.15-8.43; P < .001).
Conclusion: After the peak hazard of death, a consistently high hazard remains, but it is lower than in the peak period. The progression hazard peak is earlier, and then hazard consistently declines. The rate of dying after disease progression is about 6.59 times the rate for nonprogressors, suggesting that progression-free survival may be a relevant clinical endpoint.
Keywords: glioblastoma; hazard of death; hazard of failure (progression or death).
© The Author(s) 2015. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
References
-
- CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2007. Source: Central Brain Tumor Registry of the United States, Hinsdale, IL: website: www.cbtrus.org; 2011.
-
- Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;3595:492–507. - PubMed
-
- Lawless J. Statistical Models and Methods for Lifetime Data. New York: Wiley; 1982.
-
- Aalen O, Gjessing H. Understanding the shape of the hazard rate: a process point of view. Stat Sci. 2011;16(1):1–22.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials