Vesicular stomatitis virus enables gene transfer and transsynaptic tracing in a wide range of organisms
- PMID: 25688551
- PMCID: PMC4458151
- DOI: 10.1002/cne.23761
Vesicular stomatitis virus enables gene transfer and transsynaptic tracing in a wide range of organisms
Abstract
Current limitations in technology have prevented an extensive analysis of the connections among neurons, particularly within nonmammalian organisms. We developed a transsynaptic viral tracer originally for use in mice, and then tested its utility in a broader range of organisms. By engineering the vesicular stomatitis virus (VSV) to encode a fluorophore and either the rabies virus glycoprotein (RABV-G) or its own glycoprotein (VSV-G), we created viruses that can transsynaptically label neuronal circuits in either the retrograde or anterograde direction, respectively. The vectors were investigated for their utility as polysynaptic tracers of chicken and zebrafish visual pathways. They showed patterns of connectivity consistent with previously characterized visual system connections, and revealed several potentially novel connections. Further, these vectors were shown to infect neurons in several other vertebrates, including Old and New World monkeys, seahorses, axolotls, and Xenopus. They were also shown to infect two invertebrates, Drosophila melanogaster, and the box jellyfish, Tripedalia cystophora, a species previously intractable for gene transfer, although no clear evidence of transsynaptic spread was observed in these species. These vectors provide a starting point for transsynaptic tracing in most vertebrates, and are also excellent candidates for gene transfer in organisms that have been refractory to other methods.
Keywords: AB_10562207; AB_531908; AB_591819; RRID: AB_10053281; SciRes_000161; VSV; anterograde; centrifugal; in vivo; polysynaptic; retina; retrograde; transsynaptic; visual pathways.
© 2015 Wiley Periodicals, Inc.
Figures
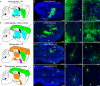
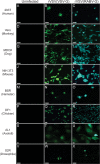

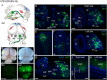
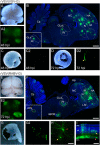
Similar articles
-
Anterograde or Retrograde Transsynaptic Circuit Tracing in Vertebrates with Vesicular Stomatitis Virus Vectors.Curr Protoc Neurosci. 2016 Jan 4;74:1.26.1-1.26.27. doi: 10.1002/0471142301.ns0126s74. Curr Protoc Neurosci. 2016. PMID: 26729030 Free PMC article.
-
Vesicular stomatitis virus with the rabies virus glycoprotein directs retrograde transsynaptic transport among neurons in vivo.Front Neural Circuits. 2013 Feb 7;7:11. doi: 10.3389/fncir.2013.00011. eCollection 2013. Front Neural Circuits. 2013. PMID: 23403489 Free PMC article.
-
Anterograde or retrograde transsynaptic labeling of CNS neurons with vesicular stomatitis virus vectors.Proc Natl Acad Sci U S A. 2011 Sep 13;108(37):15414-9. doi: 10.1073/pnas.1110854108. Epub 2011 Aug 8. Proc Natl Acad Sci U S A. 2011. PMID: 21825165 Free PMC article.
-
G gene-deficient single-round rabies viruses for neuronal circuit analysis.Virus Res. 2016 May 2;216:41-54. doi: 10.1016/j.virusres.2015.05.023. Epub 2015 Jun 8. Virus Res. 2016. PMID: 26065596 Review.
-
Gene transfer in the nervous system and implications for transsynaptic neuronal tracing.Expert Opin Biol Ther. 2010 May;10(5):763-72. doi: 10.1517/14712591003796538. Expert Opin Biol Ther. 2010. PMID: 20367126 Free PMC article. Review.
Cited by
-
The amazing and anomalous axolotls as scientific models.Dev Dyn. 2022 Jun;251(6):922-933. doi: 10.1002/dvdy.470. Epub 2022 Apr 1. Dev Dyn. 2022. PMID: 35322911 Free PMC article.
-
Thalamostriatal and cerebellothalamic pathways in a songbird, the Bengalese finch.J Comp Neurol. 2018 Jun 15;526(9):1550-1570. doi: 10.1002/cne.24428. Epub 2018 Apr 6. J Comp Neurol. 2018. PMID: 29520771 Free PMC article.
-
Zebrafish as a Translational Model: An Experimental Alternative to Study the Mechanisms Involved in Anosmia and Possible Neurodegenerative Aspects of COVID-19?eNeuro. 2021 Jun 2;8(3):ENEURO.0027-21.2021. doi: 10.1523/ENEURO.0027-21.2021. Print 2021 May-Jun. eNeuro. 2021. PMID: 33952614 Free PMC article. Review.
-
Single-Cell Analysis of Neuroinflammatory Responses Following Intracranial Injection of G-Deleted Rabies Viruses.Front Cell Neurosci. 2020 Mar 20;14:65. doi: 10.3389/fncel.2020.00065. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32265666 Free PMC article.
-
Trans-synaptic Neural Circuit-Tracing with Neurotropic Viruses.Neurosci Bull. 2019 Oct;35(5):909-920. doi: 10.1007/s12264-019-00374-9. Epub 2019 Apr 19. Neurosci Bull. 2019. PMID: 31004271 Free PMC article. Review.
References
-
- Albin RL, Young AB, Penney JB. 1995. The functional anatomy of disorders of the basal ganglia. Trends Neurosci 18: 63–64. - PubMed
-
- Alford SC, Wu J, Zhao Y, Campbell RE, Knopfel T. 2013. Optogenetic reporters. Biol Cell 105: 14–29. - PubMed
-
- Amirache F, Levy C, Costa C, Mangeot PE, Torbett BE, Wang CX, Negre D, Cosset FL, Verhoeyen E. 2014. Mystery solved: VSV‐G‐LVs do not allow efficient gene transfer into unstimulated T cells, B cells, and HSCs because they lack the LDL receptor. Blood 123: 1422–1424. - PubMed
-
- Astic L, Saucier D, Coulon P, Lafay F, Flamand A. 1993. The CVS strain of rabies virus as transneuronal tracer in the olfactory system of mice. Brain Res 619: 146–156. - PubMed
-
- Baskerville A, Lloyd G. 1977. Experimental infection of monkeys with Herpesvirus suis (Aujeszky's‐disease virus). J Med Microbiol 10: 139–144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HD067140/HD/NICHD NIH HHS/United States
- EY12196/EY/NEI NIH HHS/United States
- NS083848/NS/NINDS NIH HHS/United States
- NS068012/NS/NINDS NIH HHS/United States
- F31 NS068012/NS/NINDS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01 EY011379/EY/NEI NIH HHS/United States
- R01 EY024844/EY/NEI NIH HHS/United States
- AG041582/AG/NIA NIH HHS/United States
- T32 EY007145/EY/NEI NIH HHS/United States
- EY023911/EY/NEI NIH HHS/United States
- EY024844/EY/NEI NIH HHS/United States
- P30 EY012196/EY/NEI NIH HHS/United States
- F31 AG041582/AG/NIA NIH HHS/United States
- HD067140/HD/NICHD NIH HHS/United States
- EY7145/EY/NEI NIH HHS/United States
- R01 NS083848/NS/NINDS NIH HHS/United States
- F32 EY023911/EY/NEI NIH HHS/United States
- EY11379/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

