Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding
- PMID: 25688564
- PMCID: PMC4362204
- DOI: 10.7554/eLife.05242
Uni-directional ciliary membrane protein trafficking by a cytoplasmic retrograde IFT motor and ciliary ectosome shedding
Abstract
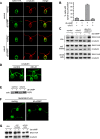
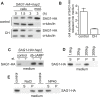
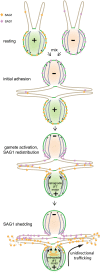
The role of the primary cilium in key signaling pathways depends on dynamic regulation of ciliary membrane protein composition, yet we know little about the motors or membrane events that regulate ciliary membrane protein trafficking in existing organelles. Recently, we showed that cilium-generated signaling in Chlamydomonas induced rapid, anterograde IFT-independent, cytoplasmic microtubule-dependent redistribution of the membrane polypeptide, SAG1-C65, from the plasma membrane to the periciliary region and the ciliary membrane. Here, we report that the retrograde IFT motor, cytoplasmic dynein 1b, is required in the cytoplasm for this rapid redistribution. Furthermore, signaling-induced trafficking of SAG1-C65 into cilia is unidirectional and the entire complement of cellular SAG1-C65 is shed during signaling and can be recovered in the form of ciliary ectosomes that retain signal-inducing activity. Thus, during signaling, cells regulate ciliary membrane protein composition through cytoplasmic action of the retrograde IFT motor and shedding of ciliary ectosomes.
Keywords: Chlamydomonas; cell biology; ciliary ectosomes; ciliary signaling; cytoplasmic dynein; intraflagellar transport; membrane protein trafficking.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures



References
-
- Bakeberg JL, Tammachote R, Woollard JR, Hogan MC, Tuan HF, Li M, van Deursen JM, Wu Y, Huang BQ, Torres VE, Harris PC, Ward CJ. Epitope-tagged Pkhd1 tracks the processing, secretion, and localization of fibrocystin. Journal of the American Society of Nephrology. 2011;22:2266–2277. doi: 10.1681/ASN.2010111173. - DOI - PMC - PubMed
-
- Cao M, Meng D, Wang L, Bei S, Snell WJ, Pan J. Activation loop phosphorylation of a protein kinase is a molecular marker of organelle size that dynamically reports flagellar length. Proceedings of the National Academy of Sciences of USA. 2013;110:12337–12342. doi: 10.1073/pnas.1302364110. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

