Investigating the role of RIO protein kinases in Caenorhabditis elegans
- PMID: 25688864
- PMCID: PMC4331490
- DOI: 10.1371/journal.pone.0117444
Investigating the role of RIO protein kinases in Caenorhabditis elegans
Erratum in
-
Correction: Investigating the Role of RIO Protein Kinases in Caenorhabditis elegans.PLoS One. 2016 May 18;11(5):e0156191. doi: 10.1371/journal.pone.0156191. eCollection 2016. PLoS One. 2016. PMID: 27192152 Free PMC article.
Abstract
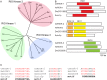
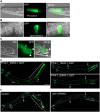
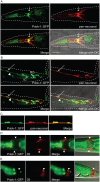
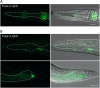
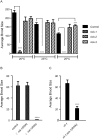
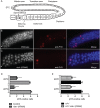
RIO protein kinases (RIOKs) are a relatively conserved family of enzymes implicated in cell cycle control and ribosomal RNA processing. Despite their functional importance, they remain a poorly understood group of kinases in multicellular organisms. Here, we show that the C. elegans genome contains one member of each of the three RIOK sub-families and that each of the genes coding for them has a unique tissue expression pattern. Our analysis showed that the gene encoding RIOK-1 (riok-1) was broadly and strongly expressed. Interestingly, the intestinal expression of riok-1 was dependent upon two putative binding sites for the oxidative and xenobiotic stress response transcription factor SKN-1. RNA interference (RNAi)-mediated knock down of riok-1 resulted in germline defects, including defects in germ line stem cell proliferation, oocyte maturation and the production of endomitotic oocytes. Taken together, our findings indicate new functions for RIOK-1 in post mitotic tissues and in reproduction.
Conflict of interest statement
Figures

References
-
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S (2002) The protein kinase complement of the human genome. Science 298: 1912–1934. - PubMed
-
- Manning G (2005) Genomic overview of Protein Kinases (December 13, 2005), WormBook, ed. The C. elegans Research Community, WormBook, Available: http://www.wormbook.org. - PMC - PubMed
-
- Hanks SK, Hunter T (1995) Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J 9: 576–596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

