Isolation of 10 cyclosporine metabolites from human bile
- PMID: 2568911
- PMCID: PMC3154783
Isolation of 10 cyclosporine metabolites from human bile
Abstract
Ten metabolites of cyclosporine were isolate from the ethyl ether extract of bile from four liver transplant patients receiving cyclosporine. Two of the metabolites were unique and previously unidentified. Liquid-liquid partitioning into diethyl ether with subsequent defatting with n-hexane was used for the initial extraction from bile. Separation of the individual metabolites (A-J) was performed using a Sephadex LH-20 column and a gradient high performance liquid chromatographic method. The molecular weights of the isolated metabolites were determined by fast atom bombardment/mass spectrometry. Gas chromatography with mass spectrometric amino acid analysis was also used to identify the amino acid composition and the hydroxylation position of metabolites A, B, C, D, and G. Proton nuclear magnetic resonance spectra were utilized to distinguish the chemical shifts of N-CH3 singlets and NH doublets of metabolites A, B, C, and D. Metabolites A, E, F, H, I, and J were reported previously in human urine and animal bile. Metabolites C and D are dihydroxylated compounds which cannot be clearly described as previously isolated compounds. Metabolites B and G are novel metabolites with a mass fragment which corresponded to a loss of 131 Da from the protonated molecular ion (MH+) in the fast atom bombardment/mass spectrometry, suggesting that the double bond in amino acid 1 has been modified. Metabolites B and G were primarily isolated from the bile of one of the liver transplant patients which contained abnormally high concentrations of these two metabolites. The method described is an efficient procedure for isolating milligram quantities of the major metabolites with greater than 95% purity.
Figures
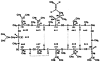
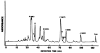

Similar articles
-
Pharmacokinetics and biotransformation of the cyclosporine metabolite M-17 in the rabbit.Drug Metab Dispos. 1990 Mar-Apr;18(2):226-30. Drug Metab Dispos. 1990. PMID: 1971578
-
Isolation, purification and structure elucidation of cyclosporin A metabolites in rabbit and man.Biomed Environ Mass Spectrom. 1989 Jan;18(1):48-56. doi: 10.1002/bms.1200180107. Biomed Environ Mass Spectrom. 1989. PMID: 2706369
-
Investigations on the metabolic pathways of cyclosporine: I. Excretion of cyclosporine and its metabolites in human bile--isolation of 12 new cyclosporine metabolites.Xenobiotica. 1991 Sep;21(9):1185-98. doi: 10.3109/00498259109039559. Xenobiotica. 1991. PMID: 1788987
-
The clinical significance of cyclosporine metabolites.Clin Biochem. 1991 Feb;24(1):23-35. doi: 10.1016/0009-9120(91)90126-y. Clin Biochem. 1991. PMID: 2060129 Review.
-
Identification of metabolism pathways of anticancer drugs by high-pressure liquid chromatography in combination with field desorption mass spectrometry.Arzneimittelforschung. 1982;32(9):995-1012. Arzneimittelforschung. 1982. PMID: 6756419 Review.
Cited by
-
Pharmacokinetic drug interactions with cyclosporin (Part II).Clin Pharmacokinet. 1990 Nov;19(5):400-15. doi: 10.2165/00003088-199019050-00004. Clin Pharmacokinet. 1990. PMID: 2268987 Review.
-
Pharmacometabolomics Enables Real-World Drug Metabolism Sciences.Metabolites. 2025 Jan 10;15(1):39. doi: 10.3390/metabo15010039. Metabolites. 2025. PMID: 39852382 Free PMC article.
-
Population pharmacokinetics and Bayesian estimation of cyclosporine in a Tunisian population of hematopoietic stem cell transplant recipient.Eur J Clin Pharmacol. 2012 Nov;68(11):1517-24. doi: 10.1007/s00228-012-1275-9. Epub 2012 Apr 15. Eur J Clin Pharmacol. 2012. PMID: 22527344
-
Human cytochrome P450 3A4: enzymatic properties of a purified recombinant fusion protein containing NADPH-P450 reductase.Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11748-52. doi: 10.1073/pnas.90.24.11748. Proc Natl Acad Sci U S A. 1993. PMID: 8265621 Free PMC article.
-
Des3PI: a fragment-based approach to design cyclic peptides targeting protein-protein interactions.J Comput Aided Mol Des. 2022 Aug;36(8):605-621. doi: 10.1007/s10822-022-00468-z. Epub 2022 Aug 6. J Comput Aided Mol Des. 2022. PMID: 35932404
References
-
- Dreyfuss M, Hani E, Hofmann H, Kobel H, Pachc W, Tscherter H. Cyclosporin A and C. New metabolites from Trichoderma polysporum (Link ex Pen.) Rifai. Eur J Appl Microbiol. 1976;3:125–133.
-
- Kahan BD. Cyclosporine. The agent and its action. Transplant Proc. 1985;17:5–18. - PubMed
-
- Borel JF. Editorial: Cyclosporin and its future. In: Borel JF, editor. Progress in Allergy. Vol. 38. Karger; New York: 1986. pp. 9–18. Cyclosporin. - PubMed
-
- Maurer G, Loosli HR, Schreier E, Keller B. Disposition of cyclosporine in several animal species and man. I. Structural elucidation of its metabolites. Drug Metab Dispos. 1984;12:120–126. - PubMed
-
- Freed BM, Rosano TG, Lempert N. In vitro immunosuppressive properties of cyclosporine metabolites. Transplantation. 1987;43:123–127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
