RNAi-Mediated CCR5 Knockdown Provides HIV-1 Resistance to Memory T Cells in Humanized BLT Mice
- PMID: 25689223
- PMCID: PMC4345313
- DOI: 10.1038/mtna.2015.3
RNAi-Mediated CCR5 Knockdown Provides HIV-1 Resistance to Memory T Cells in Humanized BLT Mice
Abstract
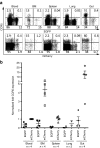
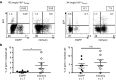
Transplantation of hematopoietic stem/progenitor cells (HSPC) modified with a lentiviral vector bearing a potent nontoxic short hairpin RNA (sh1005) directed to the HIV coreceptor CCR5 is capable of continuously producing CCR5 downregulated CD4+ T lymphocytes. Here, we characterized HIV-1 resistance of the sh1005-modified CD4+ T lymphocytes in vivo in humanized bone marrow/liver/thymus (hu BLT) mice. The sh1005-modified CD4+ T lymphocytes were positively selected in CCR5-tropic HIV-1-challenged mice. The sh1005-modified memory CD4+ T lymphocytes (the primary target of CCR5-tropic HIV-1) expressing sh1005 were maintained in lymphoid tissues in CCR5-tropic HIV-1-challenged mice. Frequencies of HIV-1 p24 expressing cells were significantly reduced in the sh1005-modified splenocytes by ex vivo cell stimulation confirming that CCR5 downregulated sh1005 modified cells are protected from viral infection. These results demonstrate that stable CCR5 downregulation through genetic modification of human HSPC by lentivirally delivered sh1005 is highly effective in providing HIV-1 resistance. Our results provide in vivo evidence in a relevant small animal model that sh1005 is a potent early-step anti-HIV reagent that has potential as a novel anti-HIV-1 HSPC gene therapeutic reagent for human applications.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

