Krüppel-like factor 6 regulates mitochondrial function in the kidney
- PMID: 25689250
- PMCID: PMC4362257
- DOI: 10.1172/JCI77084
Krüppel-like factor 6 regulates mitochondrial function in the kidney
Abstract
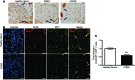
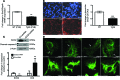
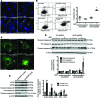
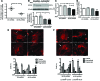
Maintenance of mitochondrial structure and function is critical for preventing podocyte apoptosis and eventual glomerulosclerosis in the kidney; however, the transcription factors that regulate mitochondrial function in podocyte injury remain to be identified. Here, we identified Krüppel-like factor 6 (KLF6), a zinc finger domain transcription factor, as an essential regulator of mitochondrial function in podocyte apoptosis. We observed that podocyte-specific deletion of Klf6 increased the susceptibility of a resistant mouse strain to adriamycin-induced (ADR-induced) focal segmental glomerulosclerosis (FSGS). KLF6 expression was induced early in response to ADR in mice and cultured human podocytes, and prevented mitochondrial dysfunction and activation of intrinsic apoptotic pathways in these podocytes. Promoter analysis and chromatin immunoprecipitation studies revealed that putative KLF6 transcriptional binding sites are present in the promoter of the mitochondrial cytochrome c oxidase assembly gene (SCO2), which is critical for preventing cytochrome c release and activation of the intrinsic apoptotic pathway. Additionally, KLF6 expression was reduced in podocytes from HIV-1 transgenic mice as well as in renal biopsies from patients with HIV-associated nephropathy (HIVAN) and FSGS. Together, these findings indicate that KLF6-dependent regulation of the cytochrome c oxidase assembly gene is critical for maintaining mitochondrial function and preventing podocyte apoptosis.
Figures


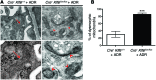
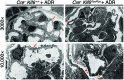
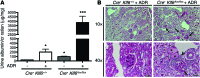
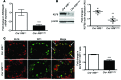

Comment in
-
Loss of Krüppel-like factor 6 cripples podocyte mitochondrial function.J Clin Invest. 2015 Mar 2;125(3):968-71. doi: 10.1172/JCI80280. Epub 2015 Feb 17. J Clin Invest. 2015. PMID: 25689255 Free PMC article.
-
Renal injury: KLF6 protects injured podocytes.Nat Rev Nephrol. 2015 Apr;11(4):197. doi: 10.1038/nrneph.2015.29. Epub 2015 Mar 10. Nat Rev Nephrol. 2015. PMID: 25752836 No abstract available.
References
-
- Holthofer H, et al. Altered gene expression and functions of mitochondria in human nephrotic syndrome. FASEB J. 1999;13(3):523–532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

