Efficacy and safety of conversion to monotherapy with eslicarbazepine acetate in adults with uncontrolled partial-onset seizures: a randomized historical-control phase III study based in North America
- PMID: 25689448
- PMCID: PMC5016771
- DOI: 10.1111/epi.12934
Efficacy and safety of conversion to monotherapy with eslicarbazepine acetate in adults with uncontrolled partial-onset seizures: a randomized historical-control phase III study based in North America
Erratum in
-
Erratum.Epilepsia. 2016 Jul;57(7):1203. doi: 10.1111/epi.13462. Epub 2016 Jun 19. Epilepsia. 2016. PMID: 27381378 Free PMC article. No abstract available.
Abstract
Objective: To assess the efficacy and safety of eslicarbazepine acetate (ESL) as monotherapy in North American patients with partial-onset seizures (POS).
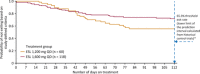
Methods: This multicenter, randomized, double-blind "withdrawal to monotherapy" study used historical control data as the comparator. Adults with POS medically uncontrolled by one to two antiepileptic drugs gradually converted to ESL monotherapy. Following an 8-week baseline period, patients were randomized 2:1 to receive ESL 1,600 mg (n = 128) or 1,200 mg QD (n = 65) for 18 weeks. The primary end point was the proportion of patients meeting predefined exit criteria (signifying worsening seizure control). Treatment was considered effective if the 95% upper confidence limit (UCL) for the Kaplan-Meier estimated exit rate was lower than the exit rate threshold calculated from the historical control (65.3%).
Results: Kaplan-Meier estimated exit rates were: ESL 1,600 mg, 28.7% (95% CI 21.2-38.1%) and 1,200 mg, 44.4% (32.5-58.3%). The difference between doses was not significant (p = 0.07). For both doses, the 95% UCLs for the exit rate were ˂ 65.3%; ESL monotherapy was considered superior to the historical control. There was no statistically significant increase in the risk of study exit related to carbamazepine use. Nine (7.6%) and five patients (8.3%) remained seizure-free during the 10-week monotherapy period, while taking ESL 1,600 and 1,200 mg, respectively. The reductions in median standardized seizure frequency (seizures per 28 days) between baseline and the 18-week treatment period were: ESL 1,600 mg, 42% and 1,200 mg, 31%. Treatment-emergent adverse events (TEAEs) occurring in ≥ 10% of patients were dizziness, headache, fatigue, somnolence, nausea, and nasopharyngitis. The TEAE most frequently leading to discontinuation was hyponatremia (2.1%).
Significance: ESL was efficacious and well tolerated as monotherapy in North American patients, and led to a reduction in seizure frequency. Exit rates for ESL 1,600 and 1,200 mg QD were superior to the historical control; the difference in exit rates between doses was not statistically significant.
Keywords: Anticonvulsants; Antiepileptic drugs; Eslicarbazepine acetate; Monotherapy; Partial-onset seizures; Refractory epilepsy.
© 2015 The Authors. Epilepsia published by Wiley Periodicals, Inc. on behalf of International League Against Epilepsy.
Figures
References
-
- Perucca E. Designing clinical trials to assess antiepileptic drugs as monotherapy. CNS Drugs 2008;22:917–938. - PubMed
-
- Perucca E, Tomson T. The pharmacological treatment of epilepsy in adults. Lancet Neurol 2011;10:446–456. - PubMed
-
- Bialer M, Soares‐da‐Silva P. Pharmacokinetics and drug interactions of eslicarbazepine acetate. Epilepsia 2012;53:935–946. - PubMed
-
- Almeida L, Bialer M, Soares‐da‐Silva P. Eslicarbazepine acetate In Shorvon S, Peruca E, Engel J. (Eds) The treatment of epilepsy. Oxford: Blackwell Publishing, 2009:485–498.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical