Altered emotionality and neuronal excitability in mice lacking KCTD12, an auxiliary subunit of GABAB receptors associated with mood disorders
- PMID: 25689571
- PMCID: PMC4445757
- DOI: 10.1038/tp.2015.8
Altered emotionality and neuronal excitability in mice lacking KCTD12, an auxiliary subunit of GABAB receptors associated with mood disorders
Abstract
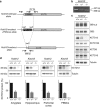
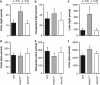
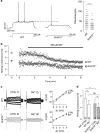
Gamma-aminobutyric acid (GABA), the major inhibitory neurotransmitter in the brain, is fundamental to brain function and implicated in the pathophysiology of several neuropsychiatric disorders. GABA activates G-protein-coupled GABAB receptors comprising principal GABAB1 and GABAB2 subunits as well as auxiliary KCTD8, 12, 12b and 16 subunits. The KCTD12 gene has been associated with bipolar disorder, major depressive disorder and schizophrenia. Here we compare Kctd12 null mutant (Kctd12(-/-)) and heterozygous (Kctd12(+/-)) with wild-type (WT) littermate mice to determine whether lack of or reduced KCTD12 expression leads to phenotypes that, extrapolating to human, could constitute endophenotypes for neuropsychiatric disorders with which KCTD12 is associated. Kctd12(-/-) mice exhibited increased fear learning but not increased memory of a discrete auditory-conditioned stimulus. Kctd12(+/-) mice showed increased activity during the inactive (light) phase of the circadian cycle relative to WT and Kctd12(-/-) mice. Electrophysiological recordings from hippocampal slices, a region of high Kctd12 expression, revealed an increased intrinsic excitability of pyramidal neurons in Kctd12(-/-) and Kctd12(+/-) mice. This is the first direct evidence for involvement of KCTD12 in determining phenotypes of emotionality, behavioral activity and neuronal excitability. This study provides empirical support for the polymorphism and expression evidence that KCTD12 confers risk for and is associated with neuropsychiatric disorders.
Figures

Similar articles
-
Behavioural endophenotypes in mice lacking the auxiliary GABAB receptor subunit KCTD16.Behav Brain Res. 2017 Jan 15;317:393-400. doi: 10.1016/j.bbr.2016.10.006. Epub 2016 Oct 4. Behav Brain Res. 2017. PMID: 27717812
-
GABAB receptor phosphorylation regulates KCTD12-induced K⁺ current desensitization.Biochem Pharmacol. 2014 Oct 1;91(3):369-79. doi: 10.1016/j.bcp.2014.07.013. Epub 2014 Jul 24. Biochem Pharmacol. 2014. PMID: 25065880 Free PMC article.
-
KCTD8 and KCTD12 Facilitate Axonal Expression of GABAB Receptors in Habenula Cholinergic Neurons.J Neurosci. 2022 Mar 2;42(9):1648-1665. doi: 10.1523/JNEUROSCI.1676-21.2021. Epub 2022 Jan 11. J Neurosci. 2022. PMID: 35017224 Free PMC article.
-
KCTD Hetero-oligomers Confer Unique Kinetic Properties on Hippocampal GABAB Receptor-Induced K+ Currents.J Neurosci. 2017 Feb 1;37(5):1162-1175. doi: 10.1523/JNEUROSCI.2181-16.2016. Epub 2016 Dec 21. J Neurosci. 2017. PMID: 28003345 Free PMC article.
-
Role of GABA(B) receptors in learning and memory and neurological disorders.Neurosci Biobehav Rev. 2016 Apr;63:1-28. doi: 10.1016/j.neubiorev.2016.01.007. Epub 2016 Jan 24. Neurosci Biobehav Rev. 2016. PMID: 26814961 Review.
Cited by
-
Lithium and GADL1 regulate glycogen synthase kinase-3 activity to modulate KCTD12 expression.Sci Rep. 2019 Jul 16;9(1):10255. doi: 10.1038/s41598-019-46655-1. Sci Rep. 2019. PMID: 31311980 Free PMC article.
-
Calpain activity is negatively regulated by a KCTD7-Cullin-3 complex via non-degradative ubiquitination.Cell Discov. 2023 Mar 24;9(1):32. doi: 10.1038/s41421-023-00533-3. Cell Discov. 2023. PMID: 36964131 Free PMC article.
-
Genome-wide association study identifies quantitative trait loci affecting cattle temperament.Zool Res. 2022 Jan 18;43(1):14-25. doi: 10.24272/j.issn.2095-8137.2021.176. Zool Res. 2022. PMID: 34766477 Free PMC article.
-
Structural complexity in the KCTD family of Cullin3-dependent E3 ubiquitin ligases.Biochem J. 2017 Nov 1;474(22):3747-3761. doi: 10.1042/BCJ20170527. Biochem J. 2017. PMID: 28963344 Free PMC article.
-
Proteomics Profiling of KAIMRC1 in Comparison to MDA-MB231 and MCF-7.Int J Mol Sci. 2020 Jun 18;21(12):4328. doi: 10.3390/ijms21124328. Int J Mol Sci. 2020. PMID: 32570693 Free PMC article.
References
-
- Padgett CL, Slesinger PA. GABAB receptor coupling to G-proteins and ion channels. Adv Pharmacol. 2010;58:123–147. - PubMed
-
- Gassmann M, Bettler B. Regulation of neuronal GABAB receptor functions by subunit composition. Nat Rev Neurosci. 2012;13:380–394. - PubMed
-
- Schwenk J, Metz M, Zolles G, Turecek R, Fritzius T, Bildl W, et al. Native GABAB receptors are heteromultimers with a family of auxiliary subunits. Nature. 2010;465:231–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

