A role for D-aspartate oxidase in schizophrenia and in schizophrenia-related symptoms induced by phencyclidine in mice
- PMID: 25689573
- PMCID: PMC4445752
- DOI: 10.1038/tp.2015.2
A role for D-aspartate oxidase in schizophrenia and in schizophrenia-related symptoms induced by phencyclidine in mice
Abstract
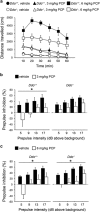
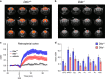
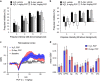
Increasing evidence points to a role for dysfunctional glutamate N-methyl-D-aspartate receptor (NMDAR) neurotransmission in schizophrenia. D-aspartate is an atypical amino acid that activates NMDARs through binding to the glutamate site on GluN2 subunits. D-aspartate is present in high amounts in the embryonic brain of mammals and rapidly decreases after birth, due to the activity of the enzyme D-aspartate oxidase (DDO). The agonistic activity exerted by D-aspartate on NMDARs and its neurodevelopmental occurrence make this D-amino acid a potential mediator for some of the NMDAR-related alterations observed in schizophrenia. Consistently, substantial reductions of D-aspartate and NMDA were recently observed in the postmortem prefrontal cortex of schizophrenic patients. Here we show that DDO mRNA expression is increased in prefrontal samples of schizophrenic patients, thus suggesting a plausible molecular event responsible for the D-aspartate imbalance previously described. To investigate whether altered D-aspartate levels can modulate schizophrenia-relevant circuits and behaviors, we also measured the psychotomimetic effects produced by the NMDAR antagonist, phencyclidine, in Ddo knockout mice (Ddo(-)(/-)), an animal model characterized by tonically increased D-aspartate levels since perinatal life. We show that Ddo(-/-) mice display a significant reduction in motor hyperactivity and prepulse inhibition deficit induced by phencyclidine, compared with controls. Furthermore, we reveal that increased levels of D-aspartate in Ddo(-/-) animals can significantly inhibit functional circuits activated by phencyclidine, and affect the development of cortico-hippocampal connectivity networks potentially involved in schizophrenia. Collectively, the present results suggest that altered D-aspartate levels can influence neurodevelopmental brain processes relevant to schizophrenia.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

