A molecular signature in the pannexin1 intracellular loop confers channel activation by the α1 adrenoreceptor in smooth muscle cells
- PMID: 25690012
- PMCID: PMC4358815
- DOI: 10.1126/scisignal.2005824
A molecular signature in the pannexin1 intracellular loop confers channel activation by the α1 adrenoreceptor in smooth muscle cells
Abstract
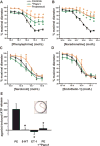
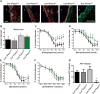
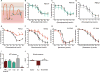
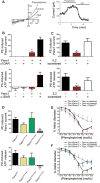
Both purinergic signaling through nucleotides such as ATP (adenosine 5'-triphosphate) and noradrenergic signaling through molecules such as norepinephrine regulate vascular tone and blood pressure. Pannexin1 (Panx1), which forms large-pore, ATP-releasing channels, is present in vascular smooth muscle cells in peripheral blood vessels and participates in noradrenergic responses. Using pharmacological approaches and mice conditionally lacking Panx1 in smooth muscle cells, we found that Panx1 contributed to vasoconstriction mediated by the α1 adrenoreceptor (α1AR), whereas vasoconstriction in response to serotonin or endothelin-1 was independent of Panx1. Analysis of the Panx1-deficient mice showed that Panx1 contributed to blood pressure regulation especially during the night cycle when sympathetic nervous activity is highest. Using mimetic peptides and site-directed mutagenesis, we identified a specific amino acid sequence in the Panx1 intracellular loop that is essential for activation by α1AR signaling. Collectively, these data describe a specific link between noradrenergic and purinergic signaling in blood pressure homeostasis.
Copyright © 2015, American Association for the Advancement of Science.
Conflict of interest statement
Figures

Comment in
-
Sympathetic vasoconstriction takes an unexpected pannexin detour.Sci Signal. 2015 Feb 17;8(364):fs4. doi: 10.1126/scisignal.aaa7312. Sci Signal. 2015. PMID: 25690011
References
-
- Burnstock G, Ralevic V. Purinergic signaling and blood vessels in health and disease. Pharmacol Rev. 2014;66:102–192. - PubMed
-
- Burnstock G. Dual control of vascular tone and remodelling by ATP released from nerves and endothelial cells. Pharmacol Rep. 2008;60:12–20. - PubMed
-
- Panchin Y, Kelmanson I, Matz M, Lukyanov K, Usman N, Lukyanov S. A ubiquitous family of putative gap junction molecules. Curr Biol. 2000;10:R473–R474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL120840/HL/NHLBI NIH HHS/United States
- DK094907/DK/NIDDK NIH HHS/United States
- R01DK088905/DK/NIDDK NIH HHS/United States
- P30 CA044579/CA/NCI NIH HHS/United States
- T32 HL094295/HL/NHLBI NIH HHS/United States
- GM107848/GM/NIGMS NIH HHS/United States
- R01 GM107848/GM/NIGMS NIH HHS/United States
- GM086457/GM/NIGMS NIH HHS/United States
- HL088554/HL/NHLBI NIH HHS/United States
- R01 DK088905/DK/NIDDK NIH HHS/United States
- HL007284/HL/NHLBI NIH HHS/United States
- R01 DK094907/DK/NIDDK NIH HHS/United States
- R01 HL088554/HL/NHLBI NIH HHS/United States
- T32 HL007284/HL/NHLBI NIH HHS/United States
- R01 GM086457/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

