Large animal in vivo evaluation of a binary blend polymer scaffold for skeletal tissue-engineering strategies; translational issues
- PMID: 25690518
- PMCID: PMC6680145
- DOI: 10.1002/term.2007
Large animal in vivo evaluation of a binary blend polymer scaffold for skeletal tissue-engineering strategies; translational issues
Abstract
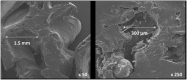
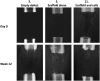
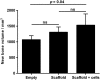
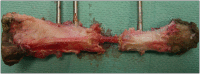
Binary blend polymers offer the opportunity to combine different desirable properties into a single scaffold, to enhance function within the field of tissue engineering. Previous in vitro and murine in vivo analysis identified a polymer blend of poly(l-lactic acid)-poly(ε-caprolactone) (PLLA:PCL 20:80) to have characteristics desirable for bone regeneration. Polymer scaffolds in combination with marrow-derived skeletal stem cells (SSCs) were implanted into mid-shaft ovine 3.5 cm tibial defects, and indices of bone regeneration were compared to groups implanted with scaffolds alone and with empty defects after 12 weeks, including micro-CT, mechanical testing and histological analysis. The critical nature of the defect was confirmed via all modalities. Both the scaffold and scaffold/SSC groups showed enhanced quantitative bone regeneration; however, this was only found to be significant in the scaffold/SSCs group (p = 0.04) and complete defect bridging was not achieved in any group. The mechanical strength was significantly less than that of contralateral control tibiae (p < 0.01) and would not be appropriate for full functional loading in a clinical setting. This study explored the hypothesis that cell therapy would enhance bone formation in a critical-sized defect compared to scaffold alone, using an external fixation construct, to bridge the scale-up gap between small animal studies and potential clinical translation. The model has proved a successful critical defect and analytical techniques have been found to be both valid and reproducible. Further work is required with both scaffold production techniques and cellular protocols in order to successfully scale-up this stem cell/binary blend polymer scaffold. © 2015 The Authors. Journal of Tissue Engineering and Regenerative Medicine published by John Wiley & Sons, Ltd.
Keywords: binary blend; bone regeneration; polymer; scaffold; skeletal stem cell.
© 2015 The Authors. Journal of Tissue Engineering and Regenerative Medicine published by John Wiley & Sons, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Cowin SC. 1989; Bone Mechanics. CRC Press: London, UK.
-
- Epari DR, Lienau J, Schell H et al. 2008; Pressure, oxygen tension and temperature in the periosteal callus during bone healing – an in vivo study in sheep. Bone 43: 734–739. - PubMed
-
- Epari DR, Schell H, Bail HJ et al. 2006; Instability prolongs the chondral phase during bone healing in sheep. Bone 38: 864–870. - PubMed
-
- Gao TJ, Tuominen TK, Lindholm TS et al. 1997; Morphological and biomechanical difference in healing in segmental tibial defects implanted with Biocoral or tricalcium phosphate cylinders. Biomaterials 18: 219–223. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

