TEFM is a potent stimulator of mitochondrial transcription elongation in vitro
- PMID: 25690892
- PMCID: PMC4357710
- DOI: 10.1093/nar/gkv105
TEFM is a potent stimulator of mitochondrial transcription elongation in vitro
Abstract
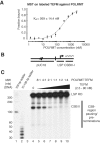
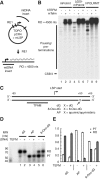
A single-subunit RNA polymerase, POLRMT, transcribes the mitochondrial genome in human cells. Recently, a factor termed as the mitochondrial transcription elongation factor, TEFM, was shown to stimulate transcription elongation in vivo, but its effect in vitro was relatively modest. In the current work, we have isolated active TEFM in recombinant form and used a reconstituted in vitro transcription system to characterize its activities. We show that TEFM strongly promotes POLRMT processivity as it dramatically stimulates the formation of longer transcripts. TEFM also abolishes premature transcription termination at conserved sequence block II, an event that has been linked to primer formation during initiation of mtDNA synthesis. We show that POLRMT pauses at a wide range of sites in a given DNA sequence. In the absence of TEFM, this leads to termination; however, the presence of TEFM abolishes this effect and aids POLRMT in continuation of transcription. Further, we show that TEFM substantially increases the POLRMT affinity to an elongation-like DNA:RNA template. In combination with previously published in vivo observations, our data establish TEFM as an essential component of the mitochondrial transcription machinery.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Falkenberg M., Larsson N.G., Gustafsson C.M. DNA replication and transcription in mammalian mitochondria. Annu. Rev. Biochem. 2007;76:679–699. - PubMed
-
- Pham X.H., Farge G., Shi Y., Gaspari M., Gustafsson C.M., Falkenberg M. Conserved sequence box II directs transcription termination and primer formation in mitochondria. J. Biol. Chem. 2006;281:24647–24652. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

