ORMDL/serine palmitoyltransferase stoichiometry determines effects of ORMDL3 expression on sphingolipid biosynthesis
- PMID: 25691431
- PMCID: PMC4373746
- DOI: 10.1194/jlr.M057539
ORMDL/serine palmitoyltransferase stoichiometry determines effects of ORMDL3 expression on sphingolipid biosynthesis
Abstract
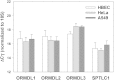
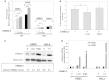
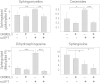
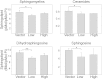
The ORM1 (Saccharomyces cerevisiae)-like proteins (ORMDLs) and their yeast orthologs, the Orms, are negative homeostatic regulators of the initiating enzyme in sphingolipid biosynthesis, serine palmitoyltransferase (SPT). Genome-wide association studies have established a strong correlation between elevated expression of the endoplasmic reticulum protein ORMDL3 and risk for childhood asthma. Here we test the notion that elevated levels of ORMDL3 decrease sphingolipid biosynthesis. This was tested in cultured human bronchial epithelial cells (HBECs) (an immortalized, but untransformed, airway epithelial cell line) and in HeLa cells (a cervical adenocarcinoma cell line). Surprisingly, elevated ORMDL3 expression did not suppress de novo biosynthesis of sphingolipids. We determined that ORMDL is expressed in functional excess relative to SPT at normal levels of expression. ORMDLs and SPT form stable complexes that are not increased by elevated ORMDL3 expression. Although sphingolipid biosynthesis was not decreased by elevated ORMDL3 expression, the steady state mass levels of all major sphingolipids were marginally decreased by low level ORMDL3 over-expression in HBECs. These data indicate that the contribution of ORMDL3 to asthma risk may involve changes in sphingolipid metabolism, but that the connection is complex.
Keywords: ORM1 (Saccharomyces cerevisiae)-like protein; Orm; asthma; ceramides; endoplasmic reticulum; enzymology/enzyme regulation; lung; sphingosine phosphate.
Figures


Similar articles
-
CRISPR/Cas9 deletion of ORMDLs reveals complexity in sphingolipid metabolism.J Lipid Res. 2021;62:100082. doi: 10.1016/j.jlr.2021.100082. Epub 2021 Apr 30. J Lipid Res. 2021. PMID: 33939982 Free PMC article.
-
The ORMDL/Orm-serine palmitoyltransferase (SPT) complex is directly regulated by ceramide: Reconstitution of SPT regulation in isolated membranes.J Biol Chem. 2019 Mar 29;294(13):5146-5156. doi: 10.1074/jbc.RA118.007291. Epub 2019 Jan 30. J Biol Chem. 2019. PMID: 30700557 Free PMC article.
-
ORMDL3 expression levels have no influence on the activity of serine palmitoyltransferase.FASEB J. 2016 Dec;30(12):4289-4300. doi: 10.1096/fj.201600639R. Epub 2016 Sep 19. FASEB J. 2016. PMID: 27645259
-
Orm/ORMDL proteins: Gate guardians and master regulators.Adv Biol Regul. 2018 Dec;70:3-18. doi: 10.1016/j.jbior.2018.08.002. Epub 2018 Aug 31. Adv Biol Regul. 2018. PMID: 30193828 Free PMC article. Review.
-
The role of ORMDL proteins, guardians of cellular sphingolipids, in asthma.Allergy. 2016 Jul;71(7):918-30. doi: 10.1111/all.12877. Epub 2016 Mar 29. Allergy. 2016. PMID: 26969910 Review.
Cited by
-
Murine endothelial serine palmitoyltransferase 1 (SPTLC1) is required for vascular development and systemic sphingolipid homeostasis.Elife. 2022 Oct 5;11:e78861. doi: 10.7554/eLife.78861. Elife. 2022. PMID: 36197001 Free PMC article.
-
Understanding the diversity of membrane lipid composition.Nat Rev Mol Cell Biol. 2018 May;19(5):281-296. doi: 10.1038/nrm.2017.138. Epub 2018 Feb 7. Nat Rev Mol Cell Biol. 2018. PMID: 29410529 Review.
-
Ceramide sensing by human SPT-ORMDL complex for establishing sphingolipid homeostasis.Nat Commun. 2023 Jun 13;14(1):3475. doi: 10.1038/s41467-023-39274-y. Nat Commun. 2023. PMID: 37308477 Free PMC article.
-
Data correlations between gender, cytomegalovirus infection and T cells, NK cells, and soluble immune mediators in elderly humans.Data Brief. 2016 Jun 15;8:536-44. doi: 10.1016/j.dib.2016.06.006. eCollection 2016 Sep. Data Brief. 2016. PMID: 27508213 Free PMC article.
-
ORMDL proteins regulate ceramide levels during sterile inflammation.J Lipid Res. 2016 Aug;57(8):1412-22. doi: 10.1194/jlr.M065920. Epub 2016 Jun 16. J Lipid Res. 2016. PMID: 27313060 Free PMC article.
References
-
- Moffatt M. F., Kabesch M., Liang L., Dixon A. L., Strachan D., Heath S., Depner M., von Berg A., Bufe A., Rietschel E., et al. 2007. Genetic variants regulating ORMDL3 expression contribute to the risk of childhood asthma. Nature. 448: 470–473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

