Novel components of the Toxoplasma inner membrane complex revealed by BioID
- PMID: 25691595
- PMCID: PMC4337574
- DOI: 10.1128/mBio.02357-14
Novel components of the Toxoplasma inner membrane complex revealed by BioID
Abstract
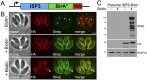
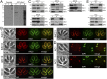
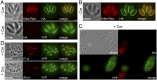
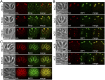
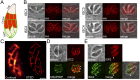
The inner membrane complex (IMC) of Toxoplasma gondii is a peripheral membrane system that is composed of flattened alveolar sacs that underlie the plasma membrane, coupled to a supporting cytoskeletal network. The IMC plays important roles in parasite replication, motility, and host cell invasion. Despite these central roles in the biology of the parasite, the proteins that constitute the IMC are largely unknown. In this study, we have adapted a technique named proximity-dependent biotin identification (BioID) for use in T. gondii to identify novel components of the IMC. Using IMC proteins in both the alveoli and the cytoskeletal network as bait, we have uncovered a total of 19 new IMC proteins in both of these suborganellar compartments, two of which we functionally evaluate by gene knockout. Importantly, labeling of IMC proteins using this approach has revealed a group of proteins that localize to the sutures of the alveolar sacs that have been seen in their entirety in Toxoplasma species only by freeze fracture electron microscopy. Collectively, our study greatly expands the repertoire of known proteins in the IMC and experimentally validates BioID as a strategy for discovering novel constituents of specific cellular compartments of T. gondii.
Importance: The identification of binding partners is critical for determining protein function within cellular compartments. However, discovery of protein-protein interactions within membrane or cytoskeletal compartments is challenging, particularly for transient or unstable interactions that are often disrupted by experimental manipulation of these compartments. To circumvent these problems, we adapted an in vivo biotinylation technique called BioID for Toxoplasma species to identify binding partners and proximal proteins within native cellular environments. We used BioID to identify 19 novel proteins in the parasite IMC, an organelle consisting of fused membrane sacs and an underlying cytoskeleton, whose protein composition is largely unknown. We also demonstrate the power of BioID for targeted discovery of proteins within specific compartments, such as the IMC cytoskeleton. In addition, we uncovered a new group of proteins localizing to the alveolar sutures of the IMC. BioID promises to reveal new insights on protein constituents and interactions within cellular compartments of Toxoplasma.
Copyright © 2015 Chen et al.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
