Raltegravir permeability across blood-tissue barriers and the potential role of drug efflux transporters
- PMID: 25691630
- PMCID: PMC4394805
- DOI: 10.1128/AAC.04594-14
Raltegravir permeability across blood-tissue barriers and the potential role of drug efflux transporters
Abstract
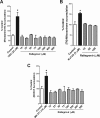
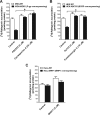
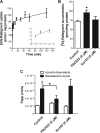
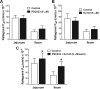
The objectives of this study were to investigate raltegravir transport across several blood-tissue barrier models and the potential interactions with drug efflux transporters. Raltegravir uptake, accumulation, and permeability were evaluated in vitro in (i) P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), multidrug resistance-associated protein 1 (MRP1), or MRP4-overexpressing MDA-MDR1 (P-gp), HEK-ABCG2, HeLa-MRP1, or HEK-MRP4 cells, respectively; (ii) cell culture systems of the human blood-brain (hCMEC/D3), mouse blood-testicular (TM4), and human blood-intestinal (Caco-2) barriers; and (iii) rat jejunum and ileum segments using an in situ single-pass intestinal perfusion model. [(3)H]Raltegravir accumulation by MDA-MDR1 (P-gp) and HEK-ABCG2-overexpressing cells was significantly enhanced in the presence of PSC833 {6-[(2S,4R,6E)-4-methyl-2-(methylamino)-3-oxo-6-octenoic acid]-7-L-valine-cyclosporine}, a P-gp inhibitor, or Ko143 [(3S,6S,12aS)-1,2,3,4,6,7,12,12a-octahydro-9-methoxy-6-(2-methylpropyl)-1,4-dioxopyrazino[1',2':1,6]pyrido[3,4-b]indole-3-propanoic acid 1,1-dimethylethyl ester], a BCRP inhibitor, suggesting the inhibition of a P-gp- or BCRP-mediated efflux process, respectively. Furthermore, [(3)H]raltegravir accumulation by human cerebral microvessel endothelial hCMEC/D3 and mouse Sertoli TM4 cells was significantly increased by PSC833 and Ko143. In human intestinal Caco-2 cells grown on Transwell filters, PSC833, but not Ko143, significantly decreased the [(3)H]raltegravir efflux ratios. In rat intestinal segments, [(3)H]raltegravir in situ permeability was significantly enhanced by the concurrent administration of PSC833 and Ko143. In contrast, in the transporter inhibition assays, raltegravir (10 to 500 μM) did not increase the accumulation of substrate for P-gp (rhodamine-6G), BCRP ([(3)H]mitoxantrone), or MRP1 [2',7'-bis(2-carboxyethyl)-5(6)-carboxyfluorescein (BCECF)] by MDA-MDR1 (P-gp)-, HEK-ABCG2-, or HeLa-MRP1-overexpressing cells, respectively. Our data suggest that raltegravir is a substrate but not an inhibitor of the drug efflux transporters P-gp and BCRP. These transporters might play a role in the restriction of raltegravir permeability across the blood-brain, blood-testicular, and blood-intestinal barriers, potentially contributing to its low tissue concentrations and/or low oral bioavailability observed in the clinic setting.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Expression of ATP-binding cassette membrane transporters in rodent and human sertoli cells: relevance to the permeability of antiretroviral therapy at the blood-testis barrier.J Pharmacol Exp Ther. 2012 Jan;340(1):96-108. doi: 10.1124/jpet.111.186916. Epub 2011 Oct 11. J Pharmacol Exp Ther. 2012. PMID: 21990609
-
Cerebrospinal fluid can be used as a surrogate to assess brain exposures of breast cancer resistance protein and P-glycoprotein substrates.Drug Metab Dispos. 2012 Apr;40(4):779-87. doi: 10.1124/dmd.111.043703. Epub 2012 Jan 19. Drug Metab Dispos. 2012. PMID: 22266779
-
Role of P-glycoprotein in the efflux of raltegravir from human intestinal cells and CD4+ T-cells as an interaction target for anti-HIV agents.Biochem Biophys Res Commun. 2013 Sep 20;439(2):221-7. doi: 10.1016/j.bbrc.2013.08.054. Epub 2013 Aug 24. Biochem Biophys Res Commun. 2013. PMID: 23981805
-
11C-Labeled rhodamine-123.2012 Nov 19 [updated 2012 Dec 27]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2012 Nov 19 [updated 2012 Dec 27]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 23285492 Free Books & Documents. Review.
-
Molecular pharmacology of ABCG2 and its role in chemoresistance.Mol Pharmacol. 2013 Nov;84(5):655-69. doi: 10.1124/mol.113.088609. Epub 2013 Sep 10. Mol Pharmacol. 2013. PMID: 24021215 Review.
Cited by
-
Drug transporters in tissues and cells relevant to sexual transmission of HIV: Implications for drug delivery.J Control Release. 2015 Dec 10;219:681-696. doi: 10.1016/j.jconrel.2015.08.018. Epub 2015 Aug 13. J Control Release. 2015. PMID: 26278511 Free PMC article. Review.
-
Uptake Transporters at the Blood-Brain Barrier and Their Role in Brain Drug Disposition.Pharmaceutics. 2023 Oct 16;15(10):2473. doi: 10.3390/pharmaceutics15102473. Pharmaceutics. 2023. PMID: 37896233 Free PMC article. Review.
-
Antiretroviral Therapy and Alcohol Interactions: X-raying Testicular and Seminal Parameters Under the HAART Era.Eur J Drug Metab Pharmacokinet. 2018 Apr;43(2):121-135. doi: 10.1007/s13318-017-0438-6. Eur J Drug Metab Pharmacokinet. 2018. PMID: 28956285 Review.
-
Development and Evaluation of Nanoparticles-in-Film Technology to Achieve Extended In Vivo Exposure of MK-2048 for HIV Prevention.Polymers (Basel). 2022 Mar 16;14(6):1196. doi: 10.3390/polym14061196. Polymers (Basel). 2022. PMID: 35335526 Free PMC article.
-
How Science Is Driving Regulatory Guidances.Methods Mol Biol. 2021;2342:595-629. doi: 10.1007/978-1-0716-1554-6_19. Methods Mol Biol. 2021. PMID: 34272707
References
-
- Palella FJ Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338:853–860. - PubMed
-
- Gange SJ, Barron Y, Greenblatt RM, Anastos K, Minkoff H, Young M, Kovacs A, Cohen M, Meyer WA III, Munoz A, Women's Interagency HIV Study Collaborative Study Group. 2002. Effectiveness of highly active antiretroviral therapy among HIV-1 infected women. J Epidemiol Community Health 56:153–159. doi:10.1136/jech.56.2.153. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

