Requisite role for Nck adaptors in cardiovascular development, endothelial-to-mesenchymal transition, and directed cell migration
- PMID: 25691664
- PMCID: PMC4387216
- DOI: 10.1128/MCB.00072-15
Requisite role for Nck adaptors in cardiovascular development, endothelial-to-mesenchymal transition, and directed cell migration
Abstract
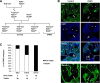
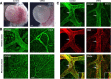
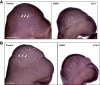
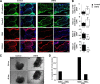
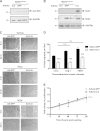
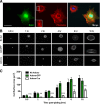
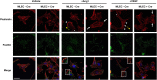
Development of the cardiovascular system is critically dependent on the ability of endothelial cells (ECs) to reorganize their intracellular actin architecture to facilitate migration, adhesion, and morphogenesis. Nck family cytoskeletal adaptors function as key mediators of actin dynamics in numerous cell types, though their role in EC biology remains largely unexplored. Here, we demonstrate an essential requirement for Nck within ECs. Mouse embryos lacking endothelial Nck1/2 expression develop extensive angiogenic defects that result in lethality at about embryonic day 10. Mutant embryos show immature vascular networks, with decreased vessel branching, aberrant perivascular cell recruitment, and reduced cardiac trabeculation. Strikingly, embryos deficient in endothelial Nck also fail to undergo the endothelial-to-mesenchymal transition (EnMT) required for cardiac valve morphogenesis, with loss of Nck disrupting expression of major EnMT markers, as well as suppressing mesenchymal outgrowth. Furthermore, we show that Nck-null ECs are unable to migrate downstream of vascular endothelial growth factor and angiopoietin-1, and they exhibit profound perturbations in cytoskeletal patterning, with disorganized cellular projections, impaired focal adhesion turnover, and disrupted actin-based signaling. Our collective findings thereby reveal a crucial role for Nck as a master regulator within the endothelium to control actin cytoskeleton organization, vascular network remodeling, and EnMT during cardiovascular development.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
