First- and second-line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: a United States-based cost-effectiveness analysis
- PMID: 25691669
- PMCID: PMC4881313
- DOI: 10.1200/JCO.2014.58.4904
First- and second-line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: a United States-based cost-effectiveness analysis
Abstract
Purpose: The addition of bevacizumab to fluorouracil-based chemotherapy is a standard of care for previously untreated metastatic colorectal cancer. Continuation of bevacizumab beyond progression is an accepted standard of care based on a 1.4-month increase in median overall survival observed in a randomized trial. No United States-based cost-effectiveness modeling analyses are currently available addressing the use of bevacizumab in metastatic colorectal cancer. Our objective was to determine the cost effectiveness of bevacizumab in the first-line setting and when continued beyond progression from the perspective of US payers.
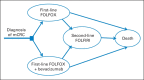
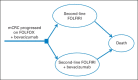
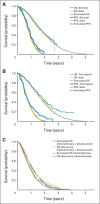
Methods: We developed two Markov models to compare the cost and effectiveness of fluorouracil, leucovorin, and oxaliplatin with or without bevacizumab in the first-line treatment and subsequent fluorouracil, leucovorin, and irinotecan with or without bevacizumab in the second-line treatment of metastatic colorectal cancer. Model robustness was addressed by univariable and probabilistic sensitivity analyses. Health outcomes were measured in life-years and quality-adjusted life-years (QALYs).
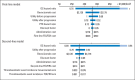
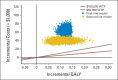
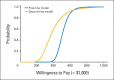
Results: Using bevacizumab in first-line therapy provided an additional 0.10 QALYs (0.14 life-years) at a cost of $59,361. The incremental cost-effectiveness ratio was $571,240 per QALY. Continuing bevacizumab beyond progression provided an additional 0.11 QALYs (0.16 life-years) at a cost of $39,209. The incremental cost-effectiveness ratio was $364,083 per QALY. In univariable sensitivity analyses, the variables with the greatest influence on the incremental cost-effectiveness ratio were bevacizumab cost, overall survival, and utility.
Conclusion: Bevacizumab provides minimal incremental benefit at high incremental cost per QALY in both the first- and second-line settings of metastatic colorectal cancer treatment.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures
Comment in
-
Can money really be no object when cancer care is the subject?J Clin Oncol. 2015 Apr 1;33(10):1093-4. doi: 10.1200/JCO.2014.60.1401. Epub 2015 Feb 17. J Clin Oncol. 2015. PMID: 25691668 No abstract available.
-
Reply to A. Messori et al, R. Bordonaro et al, and G. Fasola et al.J Clin Oncol. 2015 Nov 10;33(32):3842-3. doi: 10.1200/JCO.2015.62.9279. Epub 2015 Aug 24. J Clin Oncol. 2015. PMID: 26304876 No abstract available.
-
Bevacizumab Plus Chemotherapy Cost Effectiveness.J Clin Oncol. 2015 Nov 10;33(32):3840-1. doi: 10.1200/JCO.2015.62.0195. Epub 2015 Aug 24. J Clin Oncol. 2015. PMID: 26304880 No abstract available.
-
Balancing Clinical Progress With Economic Sustainability: In Quest of a Courageous Solution.J Clin Oncol. 2015 Nov 10;33(32):3841-2. doi: 10.1200/JCO.2015.61.9601. Epub 2015 Aug 24. J Clin Oncol. 2015. PMID: 26304899 No abstract available.
References
-
- Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin. 2014;64:104–117. - PubMed
-
- Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23–30. - PubMed
-
- Cassidy J, Clarke S, Díaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006–2012. - PubMed
-
- Hochster HS, Hart LL, Ramanathan RK, et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: Results of the TREE study. J Clin Oncol. 2008;26:3523–3529. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

