Final Report of the Intergroup Randomized Study of Combined Androgen-Deprivation Therapy Plus Radiotherapy Versus Androgen-Deprivation Therapy Alone in Locally Advanced Prostate Cancer
- PMID: 25691677
- PMCID: PMC4477786
- DOI: 10.1200/JCO.2014.57.7510
Final Report of the Intergroup Randomized Study of Combined Androgen-Deprivation Therapy Plus Radiotherapy Versus Androgen-Deprivation Therapy Alone in Locally Advanced Prostate Cancer
Abstract
Purpose: We have previously reported that radiotherapy (RT) added to androgen-deprivation therapy (ADT) improves survival in men with locally advanced prostate cancer. Here, we report the prespecified final analysis of this randomized trial.
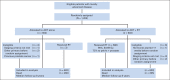
Patients and methods: NCIC Clinical Trials Group PR.3/Medical Research Council PR07/Intergroup T94-0110 was a randomized controlled trial of patients with locally advanced prostate cancer. Patients with T3-4, N0/Nx, M0 prostate cancer or T1-2 disease with either prostate-specific antigen (PSA) of more than 40 μg/L or PSA of 20 to 40 μg/L plus Gleason score of 8 to 10 were randomly assigned to lifelong ADT alone or to ADT+RT. The RT dose was 64 to 69 Gy in 35 to 39 fractions to the prostate and pelvis or prostate alone. Overall survival was compared using a log-rank test stratified for prespecified variables.
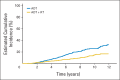
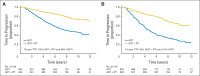
Results: One thousand two hundred five patients were randomly assigned between 1995 and 2005, 602 to ADT alone and 603 to ADT+RT. At a median follow-up time of 8 years, 465 patients had died, including 199 patients from prostate cancer. Overall survival was significantly improved in the patients allocated to ADT+RT (hazard ratio [HR], 0.70; 95% CI, 0.57 to 0.85; P < .001). Deaths from prostate cancer were significantly reduced by the addition of RT to ADT (HR, 0.46; 95% CI, 0.34 to 0.61; P < .001). Patients on ADT+RT reported a higher frequency of adverse events related to bowel toxicity, but only two of 589 patients had grade 3 or greater diarrhea at 24 months after RT.
Conclusion: This analysis demonstrates that the previously reported benefit in survival is maintained at a median follow-up of 8 years and firmly establishes the role of RT in the treatment of men with locally advanced prostate cancer.
Trial registration: ClinicalTrials.gov NCT00002633.
© 2015 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures

References
-
- Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61:1079–1092. - PubMed
-
- Ward JF, Slezak JM, Blute ML, et al. Radical prostatectomy for clinically advanced (cT3) prostate cancer since the advent of prostate-specific antigen testing: 15-year outcome. BJU Int. 2005;95:751–756. - PubMed
-
- Fellows GJ, Clark PB, Beynon LL, et al. Treatment of advanced localised prostatic cancer by orchiectomy, radiotherapy, or combined treatment: A Medical Research Council Study—Urological Cancer Working Party–Subgroup on Prostatic Cancer. Br J Urol. 1992;70:304–309. - PubMed
-
- Mason MD, Brewster S, Moffat LE, et al. Randomized trials in early prostate cancer: II. Hormone therapy and radiotherapy for locally advanced disease: A question is still unanswered. Clin Oncol (R Coll Radiol) 2000;12:215–216. - PubMed
-
- Bolla M, de Reijke TM, Van Tienhoven G, et al. Duration of androgen suppression in the treatment of prostate cancer. N Engl J Med. 2009;360:2516–2527. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

