Antisense long noncoding RNAs regulate var gene activation in the malaria parasite Plasmodium falciparum
- PMID: 25691743
- PMCID: PMC4352787
- DOI: 10.1073/pnas.1420855112
Antisense long noncoding RNAs regulate var gene activation in the malaria parasite Plasmodium falciparum
Abstract
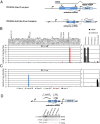
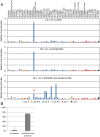
The virulence of Plasmodium falciparum, the causative agent of the deadliest form of human malaria, is attributed to its ability to evade human immunity through antigenic variation. These parasites alternate between expression of variable antigens, encoded by members of a multicopy gene family named var. Immune evasion through antigenic variation depends on tight regulation of var gene expression, ensuring that only a single var gene is expressed at a time while the rest of the family is maintained transcriptionally silent. Understanding how a single gene is chosen for activation is critical for understanding mutually exclusive expression but remains a mystery. Here, we show that antisense long noncoding RNAs (lncRNAs) initiating from var introns are associated with the single active var gene at the time in the cell cycle when the single var upstream promoter is active. We demonstrate that these antisense transcripts are incorporated into chromatin, and that expression of these antisense lncRNAs in trans triggers activation of a silent var gene in a sequence- and dose-dependent manner. On the other hand, interference with these lncRNAs using complement peptide nucleic acid molecules down-regulated the active var gene, erased the epigenetic memory, and induced expression switching. Altogether, our data provide evidence that these antisense lncRNAs play a key role in regulating var gene activation and mutually exclusive expression.
Keywords: Plasmodium falciparum; exclusive expression; malaria; noncoding RNA; var genes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Comment in
-
Parasite biology: The antisense antigen switch.Nat Rev Microbiol. 2015 Apr;13(4):188-9. doi: 10.1038/nrmicro3455. Epub 2015 Mar 2. Nat Rev Microbiol. 2015. PMID: 25730703 No abstract available.
References
-
- WHO 2010. World Malaria Report 2010 (World Health Organization, Geneva)
-
- Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415(6872):673–679. - PubMed
-
- Su XZ, et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell. 1995;82(1):89–100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

