Deficits in endogenous adenosine formation by ecto-5'-nucleotidase/CD73 impair neuromuscular transmission and immune competence in experimental autoimmune myasthenia gravis
- PMID: 25691808
- PMCID: PMC4322825
- DOI: 10.1155/2015/460610
Deficits in endogenous adenosine formation by ecto-5'-nucleotidase/CD73 impair neuromuscular transmission and immune competence in experimental autoimmune myasthenia gravis
Abstract
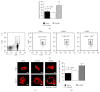
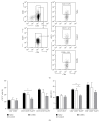
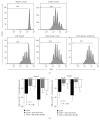
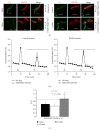
AMP dephosphorylation via ecto-5'-nucleotidase/CD73 is the rate limiting step to generate extracellular adenosine (ADO) from released adenine nucleotides. ADO, via A2A receptors (A2ARs), is a potent modulator of neuromuscular and immunological responses. The pivotal role of ecto-5'-nucleotidase/CD73, in controlling extracellular ADO formation, prompted us to investigate its role in a rat model of experimental autoimmune myasthenia gravis (EAMG). Results show that CD4(+)CD25(+)FoxP3(+) regulatory T cells express lower amounts of ecto-5'-nucleotidase/CD73 as compared to controls. Reduction of endogenous ADO formation might explain why proliferation of CD4(+) T cells failed upon blocking A2A receptors activation with ZM241385 or adenosine deaminase in EAMG animals. Deficits in ADO also contribute to neuromuscular transmission failure in EAMG rats. Rehabilitation of A2AR-mediated immune suppression and facilitation of transmitter release were observed by incubating the cells with the nucleoside precursor, AMP. These findings, together with the characteristic increase in serum adenosine deaminase activity of MG patients, strengthen our hypothesis that the adenosinergic pathway may be dysfunctional in EAMG. Given that endogenous ADO formation is balanced by ecto-5'-nucleotidase/CD73 activity and that A2ARs exert a dual role to restore use-dependent neurocompetence and immune suppression in myasthenics, we hypothesize that stimulation of the two mechanisms may have therapeutic potential in MG.
Figures

Similar articles
-
Involvement of CD73, equilibrative nucleoside transporters and inosine in rhythm and conduction disturbances mediated by adenosine A1 and A2A receptors in the developing heart.J Mol Cell Cardiol. 2013 Oct;63:14-25. doi: 10.1016/j.yjmcc.2013.06.008. Epub 2013 Jul 6. J Mol Cell Cardiol. 2013. PMID: 23837961
-
The ecto-enzymes CD73 and adenosine deaminase modulate 5'-AMP-derived adenosine in myofibroblasts of the rat small intestine.Purinergic Signal. 2018 Dec;14(4):409-421. doi: 10.1007/s11302-018-9623-6. Epub 2018 Sep 29. Purinergic Signal. 2018. PMID: 30269308 Free PMC article.
-
Adenosine signalling mediates the anti-inflammatory effects of the COX-2 inhibitor nimesulide.Biochem Pharmacol. 2016 Jul 15;112:72-81. doi: 10.1016/j.bcp.2016.05.006. Epub 2016 May 14. Biochem Pharmacol. 2016. PMID: 27188793
-
Ecto-5'-nucleotidase (CD73)-mediated extracellular adenosine production plays a critical role in hepatic fibrosis.Nucleosides Nucleotides Nucleic Acids. 2008 Jun;27(6):821-4. doi: 10.1080/15257770802146403. Nucleosides Nucleotides Nucleic Acids. 2008. PMID: 18600546 Review.
-
The Role of Extracellular Adenosine Generation in the Development of Autoimmune Diseases.Mediators Inflamm. 2018 Mar 26;2018:7019398. doi: 10.1155/2018/7019398. eCollection 2018. Mediators Inflamm. 2018. PMID: 29769837 Free PMC article. Review.
Cited by
-
Purinergic Signalling: Therapeutic Developments.Front Pharmacol. 2017 Sep 25;8:661. doi: 10.3389/fphar.2017.00661. eCollection 2017. Front Pharmacol. 2017. PMID: 28993732 Free PMC article. Review.
-
Modulatory Roles of ATP and Adenosine in Cholinergic Neuromuscular Transmission.Int J Mol Sci. 2020 Sep 3;21(17):6423. doi: 10.3390/ijms21176423. Int J Mol Sci. 2020. PMID: 32899290 Free PMC article. Review.
-
Synaptic Homeostasis and Its Immunological Disturbance in Neuromuscular Junction Disorders.Int J Mol Sci. 2017 Apr 24;18(4):896. doi: 10.3390/ijms18040896. Int J Mol Sci. 2017. PMID: 28441759 Free PMC article. Review.
-
Ectonucleotidases in Acute and Chronic Inflammation.Front Pharmacol. 2021 Feb 3;11:619458. doi: 10.3389/fphar.2020.619458. eCollection 2020. Front Pharmacol. 2021. PMID: 33613285 Free PMC article. Review.
-
Purines and Carotid Body: New Roles in Pathological Conditions.Front Pharmacol. 2017 Dec 12;8:913. doi: 10.3389/fphar.2017.00913. eCollection 2017. Front Pharmacol. 2017. PMID: 29311923 Free PMC article. Review.
References
-
- Lindstrom J. M. Acetylcholine receptors and myasthenia. Muscle & Nerve. 2000;23(4):453–477. - PubMed
-
- Correia-de-Sá P., Sebastião A. M., Ribeiro J. A. Inhibitory and excitatory effects of adenosine receptor agonists on evoked transmitter release from phrenic nerve endings of the rat. British Journal of Pharmacology. 1991;103(2):1614–1620. doi: 10.1111/j.1476-5381.1991.tb09836.x. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

