A use-dependent increase in release sites drives facilitation at calretinin-deficient cerebellar parallel-fiber synapses
- PMID: 25691858
- PMCID: PMC4315043
- DOI: 10.3389/fncel.2015.00027
A use-dependent increase in release sites drives facilitation at calretinin-deficient cerebellar parallel-fiber synapses
Abstract
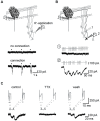
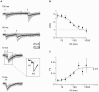
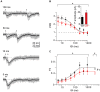
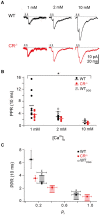
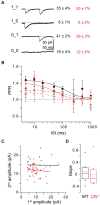
Endogenous Ca(2+)-binding proteins affect synaptic transmitter release and short-term plasticity (STP) by buffering presynaptic Ca(2+) signals. At parallel-fiber (PF)-to-Purkinje neuron (PN) synapses in the cerebellar cortex loss of calretinin (CR), the major buffer at PF terminals, results in increased presynaptic Ca(2+) transients and an almost doubling of the initial vesicular releases probability (p r). Surprisingly, however, it has been reported that loss of CR from PF synapses does not alter paired-pulse facilitation (PPF), while it affects presynaptic Ca(2+) signals as well as p r. Here, we addressed this puzzling observation by analyzing the frequency- and Ca(2+)-dependence of PPF at unitary PF-to-PN synapses of wild-type (WT) and CR-deficient (CR(-/-)) mice using paired recordings and computer simulations. Our analysis revealed that PPF in CR(-/-) is indeed smaller than in the WT, to a degree, however, that indicates that rapid vesicle replenishment and recruitment of additional release sites dominate the synaptic efficacy of the second response. These Ca(2+)-driven processes operate more effectively in the absence of CR, thereby, explaining the preservation of robust PPF in the mutants.
Keywords: calretinin; granule cells; paired recordings; paired-pulse facilitation; ready-releasable pool; short-term plasticity.
Figures
Similar articles
-
Control of Presynaptic Parallel Fiber Efficacy by Activity-Dependent Regulation of the Number of Occupied Release Sites.Front Syst Neurosci. 2019 Jul 17;13:30. doi: 10.3389/fnsys.2019.00030. eCollection 2019. Front Syst Neurosci. 2019. PMID: 31379524 Free PMC article.
-
Paired-pulse facilitation at recurrent Purkinje neuron synapses is independent of calbindin and parvalbumin during high-frequency activation.J Physiol. 2013 Jul 1;591(13):3355-70. doi: 10.1113/jphysiol.2013.254128. Epub 2013 May 13. J Physiol. 2013. PMID: 23671160 Free PMC article.
-
Differential modulation of cerebellar climbing fiber and parallel fiber synaptic responses at high pressure.J Appl Physiol (1985). 2009 Feb;106(2):729-36. doi: 10.1152/japplphysiol.90853.2008. Epub 2008 Dec 4. J Appl Physiol (1985). 2009. PMID: 19057002
-
Calcium and transmitter release.J Physiol Paris. 1993;87(1):25-36. doi: 10.1016/0928-4257(93)90021-k. J Physiol Paris. 1993. PMID: 7905762 Review.
-
Activity-dependent regulation of synaptic vesicle exocytosis and presynaptic short-term plasticity.Neurosci Res. 2011 May;70(1):16-23. doi: 10.1016/j.neures.2011.03.005. Epub 2011 Mar 29. Neurosci Res. 2011. PMID: 21453732 Review.
Cited by
-
What We Can Learn From Cumulative Numbers of Vesicular Release Events.Front Cell Neurosci. 2019 Jun 21;13:257. doi: 10.3389/fncel.2019.00257. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31293386 Free PMC article.
-
Short-term plasticity at cerebellar granule cell to molecular layer interneuron synapses expands information processing.Elife. 2019 May 13;8:e41586. doi: 10.7554/eLife.41586. Elife. 2019. PMID: 31081751 Free PMC article.
-
Calretinin-Expressing Synapses Show Improved Synaptic Efficacy with Reduced Asynchronous Release during High-Rate Activity.J Neurosci. 2022 Mar 30;42(13):2729-2742. doi: 10.1523/JNEUROSCI.1773-21.2022. Epub 2022 Feb 14. J Neurosci. 2022. PMID: 35165172 Free PMC article.
-
Control of Presynaptic Parallel Fiber Efficacy by Activity-Dependent Regulation of the Number of Occupied Release Sites.Front Syst Neurosci. 2019 Jul 17;13:30. doi: 10.3389/fnsys.2019.00030. eCollection 2019. Front Syst Neurosci. 2019. PMID: 31379524 Free PMC article.
-
Frequency-dependent mobilization of heterogeneous pools of synaptic vesicles shapes presynaptic plasticity.Elife. 2017 Oct 9;6:e28935. doi: 10.7554/eLife.28935. Elife. 2017. PMID: 28990927 Free PMC article.
References
-
- Bertram R., Sherman A., Stanley E. F. (1996). Single-domain/bound calcium hypothesis of transmitter release and facilitation. J. Neurophysiol. 75, 1919–1931. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

