Ion channelopathies in human induced pluripotent stem cell derived cardiomyocytes: a dynamic clamp study with virtual IK1
- PMID: 25691870
- PMCID: PMC4315032
- DOI: 10.3389/fphys.2015.00007
Ion channelopathies in human induced pluripotent stem cell derived cardiomyocytes: a dynamic clamp study with virtual IK1
Abstract
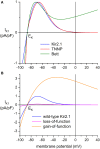
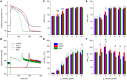
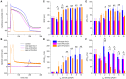
Human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs) are widely used in studying basic mechanisms of cardiac arrhythmias that are caused by ion channelopathies. Unfortunately, the action potential profile of hiPSC-CMs-and consequently the profile of individual membrane currents active during that action potential-differs substantially from that of native human cardiomyocytes, largely due to almost negligible expression of the inward rectifier potassium current (IK1). In the present study, we attempted to "normalize" the action potential profile of our hiPSC-CMs by inserting a voltage dependent in silico IK1 into our hiPSC-CMs, using the dynamic clamp configuration of the patch clamp technique. Recordings were made from single hiPSC-CMs, using the perforated patch clamp technique at physiological temperature. We assessed three different models of IK1, with different degrees of inward rectification, and systematically varied the magnitude of the inserted IK1. Also, we modified the inserted IK1 in order to assess the effects of loss- and gain-of-function mutations in the KCNJ2 gene, which encodes the Kir2.1 protein that is primarily responsible for the IK1 channel in human ventricle. For our experiments, we selected spontaneously beating hiPSC-CMs, with negligible IK1 as demonstrated in separate voltage clamp experiments, which were paced at 1 Hz. Upon addition of in silico IK1 with a peak outward density of 4-6 pA/pF, these hiPSC-CMs showed a ventricular-like action potential morphology with a stable resting membrane potential near -80 mV and a maximum upstroke velocity >150 V/s (n = 9). Proarrhythmic action potential changes were observed upon injection of both loss-of-function and gain-of-function IK1, as associated with Andersen-Tawil syndrome type 1 and short QT syndrome type 3, respectively (n = 6). We conclude that injection of in silico IK1 makes the hiPSC-CM a more reliable model for investigating mechanisms underlying cardiac arrhythmias.
Keywords: Andersen–Tawil syndrome; KCNJ2 gene; Kir2.1 protein; action potentials; cardiac ion channelopathies; inward rectifier potassium channel; patch clamp; short QT syndrome 3.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

