The regulation of muscle mass by endogenous glucocorticoids
- PMID: 25691871
- PMCID: PMC4315033
- DOI: 10.3389/fphys.2015.00012
The regulation of muscle mass by endogenous glucocorticoids
Abstract
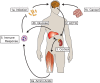
Glucocorticoids are highly conserved fundamental regulators of energy homeostasis. In response to stress in the form of perceived danger or acute inflammation, glucocorticoids are released from the adrenal gland, rapidly mobilizing energy from carbohydrate, fat and protein stores. In the case of inflammation, mobilized protein is critical for the rapid synthesis of acute phase reactants and an efficient immune response to infection. While adaptive in response to infection, chronic mobilization can lead to a profound depletion of energy stores. Skeletal muscle represents the major body store of protein, and can become substantially atrophied under conditions of chronic inflammation. Glucocorticoids elicit the atrophy of muscle by increasing the rate of protein degradation by the ubiquitin-proteasome system and autophagy lysosome system. Protein synthesis is also suppressed at the level of translational initiation, preventing the production of new myofibrillar protein. Glucocorticoids also antagonize the action of anabolic regulators such as insulin further exacerbating the loss of protein and muscle mass. The loss of muscle mass in the context of chronic disease is a key feature of cachexia and contributes substantially to morbidity and mortality. A growing body of evidence demonstrates that glucocorticoid signaling is a common mediator of wasting, irrespective of the underlying initiator or disease state. This review will highlight fundamental mechanisms of glucocorticoid signaling and detail the mechanisms of glucocorticoid-induced muscle atrophy. Additionally, the evidence for glucocorticoids as a driver of muscle wasting in numerous disease states will be discussed. Given the burden of wasting diseases and the nodal nature of glucocorticoid signaling, effective anti-glucocorticoid therapy would be a valuable clinical tool. Therefore, the progress and potential pitfalls in the development of glucocorticoid antagonists for muscle wasting will be discussed.
Keywords: HPA-axis; cachexia; catabolism; glucococorticoids; muscle; protein synthesis; skeletal; wasting.
Figures

References
-
- Akita S., Webster J., Ren S. G., Takino H., Said J., Zand O., et al. . (1995). Human and murine pituitary expression of leukemia inhibitory factor. Novel intrapituitary regulation of adrenocorticotropin hormone synthesis and secretion. J. Clin. Invest. 95, 1288–1298. 10.1172/JCI117779 - DOI - PMC - PubMed
-
- Baracos V., Rodemann H. P., Dinarello C. A., Goldberg A. L. (1983). Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N. Engl. J. Med. 308, 553–558. 10.1056/NEJM198303103081002 - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

