Surface protein mutations in chronic hepatitis B patients who received hepatitis B vaccine therapy
- PMID: 25691938
- PMCID: PMC4322145
Surface protein mutations in chronic hepatitis B patients who received hepatitis B vaccine therapy
Abstract
Objective s: The aim of this study was to determine the correlation between vaccine therapy and appearance of mutations in hepatitis B surface antigen (HBsAg)-positive chronic hepatitis B virus (HBV) patients.
Materials and methods: 16 patients received the HBV vaccine and another 16 individuals from the control group did not. The surface gene was amplified and directly sequenced from samples prior to vaccination and six months after the third dose.
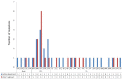
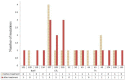
Results: Only one patient lost HBsAg. 48 and 44 amino acid mutations were found before and after vaccine therapy in the vaccine group respectively, 51 of which (55.4%) occurred in immune epitopes: 5 were in B cell, 21 in T helper (Th), and 25 in cytotoxic T-lymphocyte (CTL) epitopes. In the control group, 35 and 41 amino acid substitutions were found before and after therapy, respectively. 32 (42%) of 76 amino acid changes occurred within immune epitopes. There were no differences in age, gender, and duration of chronicity in both patient and control groups in terms of the frequency and the patterns of mutations.
Conclusion: In chronic carriers who already had HBsAg variants selected by the host-immune response, any immune stimulation by the vaccine had no effect on the chronic state of these patients or selected any remarkable escape mutants. Newer strategies should be considered based on third generation or the use of DNA vaccines or new adjuvants.
Keywords: HBsAg mutants; Hepatitis B immune epitopes; Hepatitis B vaccine.
Figures

Similar articles
-
Hepatitis B virus surface protein mutations clustered mainly in CTL immune epitopes in chronic carriers: results of an Iranian nationwide study.J Viral Hepat. 2013 Jul;20(7):494-501. doi: 10.1111/jvh.12045. Epub 2013 Apr 25. J Viral Hepat. 2013. PMID: 23730843
-
High frequency of hepatitis B virus infection in patients with beta-thalassemia receiving multiple transfusions.Vox Sang. 2003 May;84(4):292-9. doi: 10.1046/j.1423-0410.2003.00300.x. Vox Sang. 2003. PMID: 12757503
-
Hepatitis B virus surface antigen (HBsAg) mutations are rare but clustered in immune epitopes in chronic carriers from Sistan-Balouchestan Province, Iran.Arch Iran Med. 2013 Jul;16(7):385-9. Arch Iran Med. 2013. PMID: 23808774
-
Vaccine- and hepatitis B immune globulin-induced escape mutations of hepatitis B virus surface antigen.J Biomed Sci. 2001 May-Jun;8(3):237-47. doi: 10.1007/BF02256597. J Biomed Sci. 2001. PMID: 11385295 Review.
-
Hepatitis B virus vaccine breakthrough infection: surveillance of S gene mutants of HBV.Acta Virol. 2018;62(2):115-121. doi: 10.4149/av_2018_210. Acta Virol. 2018. PMID: 29895151 Review.
Cited by
-
Current progress in the development of therapeutic vaccines for chronic hepatitis B virus infection.Iran J Basic Med Sci. 2016 Jul;19(7):692-704. Iran J Basic Med Sci. 2016. PMID: 27635192 Free PMC article. Review.
References
-
- Fontaine H, Thiers V, Pol S. Hepatitis B virus genotypic resistance to lamivudine. Ann Intern Med. 1999;131:716–717. - PubMed
-
- Zoulim F, Trepo C. New antiviral agents for the therapy of chronic hepatitis B virus infection. Intervirology . 1999;42:125–144. - PubMed
-
- Bidgoli SA, Daryani NE, Motamedi M, Miri A, Poorsamimi P. Evaluation of possible risk factors of lamivudine resistance in chronic hepatitis b patients: a retrospective study in Iran. Hepat Mon. 2009; 9:171–179.
-
- Pol S, Driss F, Michel ML, Nalpas B, Berthelot P, Brechot C. Specific vaccine therapy in chronic hepatitis B infection. Lancet . 1994; 344:342. - PubMed
-
- Pol S, Michel ML, Brechot C. Immune therapy of hepatitis B virus chronic infection. Hepatology . 2000; 31:548–549. - PubMed
LinkOut - more resources
Full Text Sources
