Bioequivalence of Sandoz methylphenidate osmotic-controlled release tablet with Concerta® (Janssen-Cilag)
- PMID: 25692005
- PMCID: PMC4317218
- DOI: 10.1002/prp2.72
Bioequivalence of Sandoz methylphenidate osmotic-controlled release tablet with Concerta® (Janssen-Cilag)
Abstract
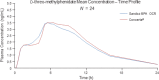
The aim was to assess the bioequivalence of Sandoz methylphenidate osmotic-controlled release (OCR) tablets (Sandoz [Methylphenidate[ MPH OCR) with Concerta®, a methylphenidate formulation indicated for the treatment of attention deficit/hyperactivity disorder (ADHD). Four open-label, randomized, single-dose, two-way crossover bioequivalence studies were conducted in healthy subjects: three fasting studies with 54-, 36- and 18-mg doses of methylphenidate, and one fed study with the 54-mg dose. The d- and l-threo-methylphenidate plasma levels were quantified using liquid chromatographic methods with tandem mass spectrometry (LC MS/MS). Bioequivalence of the formulations was accepted if the 90% geometric confidence intervals of the ratio of least-squares means of Sandoz MPH OCR to Concerta® of ln-transformed area under the curve (AUC0-t ) and C max were within the acceptance range of 80-125%. All studies met the bioequivalence criteria, and 90% geometric confidence intervals for AUC0-t and C max were within the predefined range. All plasma concentration time curves for Sandoz MPH OCR under fasting conditions showed a biphasic profile comparable with Concerta®, confirmed by bioequivalence of the partial metrics AUC0-2h, AUC2-24 h, C max(0-2 h) and C max(2-24 h). Both products were well tolerated and no relevant differences in the safety profiles were observed. It was concluded that Sandoz MPH OCR is bioequivalent to Concerta® in terms of rate and extent of absorption when administered as a single dose of one extended-release tablet of 54, 36, or 18 mg under fasting conditions and at a dose of 54 mg under fed conditions.
Keywords: ADHD; Concerta®; Sandoz; bioequivalence; extended release; methylphenidate; osmotic-controlled release.
Figures
References
-
- Hauschke D, Steinijans VW, Diletti E, Burke M. Sample size determination for bioequivalence assessment using a multiplicative model. J Pharmacokinet Biopharm. 1992;20:557–561. - PubMed
-
- Janssen-Cilag. 2013. 18 February Concerta®XL 18 mg-36 mg prolonged release tablets. Summary of product characteristics.
-
- Markowitz JS, Straughn AB, Patrick KS. Advances in the pharmacotherapy of attention-deficit–hyperactivity disorder: focus on methylphenidate formulations. Pharmacotherapy. 2003;23:1281–1299. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous