Chemical characteristics, synthetic methods, and biological potential of quinazoline and quinazolinone derivatives
- PMID: 25692041
- PMCID: PMC4321853
- DOI: 10.1155/2014/395637
Chemical characteristics, synthetic methods, and biological potential of quinazoline and quinazolinone derivatives
Abstract
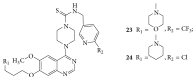
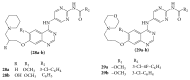
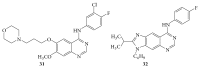
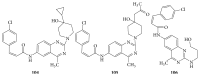
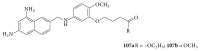
The heterocyclic fused rings quinazoline and quinazolinone have drawn a huge consideration owing to their expanded applications in the field of pharmaceutical chemistry. Quinazoline and quinazolinone are reported for their diversified biological activities and compounds with different substitutions bring together to knowledge of a target with understanding of the molecule types that might interact with the target receptors. Quinazolines and quinazolinones are considered as an important chemical for the synthesis of various physiological significance and pharmacological utilized molecules. Quinazolines and quinazolinone are a large class of biologically active compounds that exhibited broad spectrum of biological activities such as anti-HIV, anticancer, antifungal, antibacterial, antimutagenic, anticoccidial, anticonvulsant, anti-inflammatory, antidepressant, antimalarial, antioxidant, antileukemic, and antileishmanial activities and other activities. Being considered as advantaged scaffold, the alteration is made with different substituent.
Figures



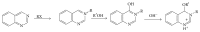
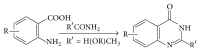
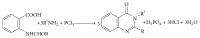
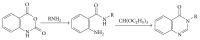

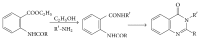





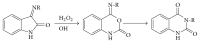
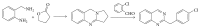

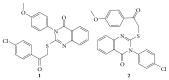

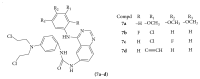
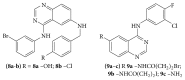
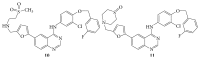
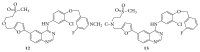

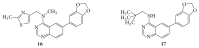

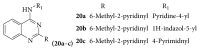
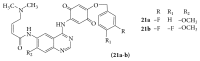
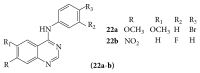


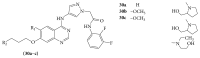
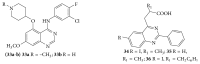
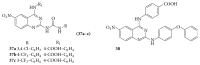
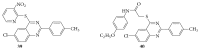
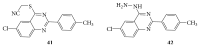
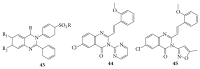
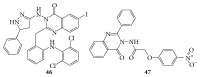
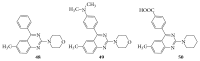

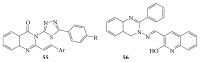
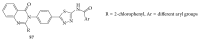

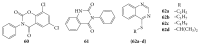

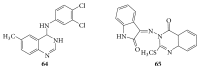

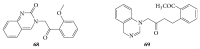
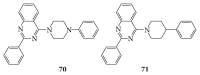
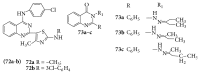


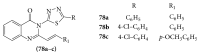

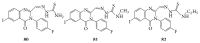
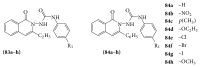
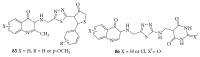
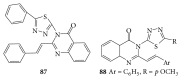
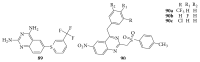
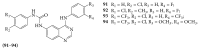
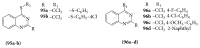
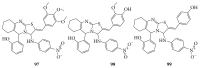
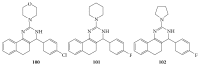
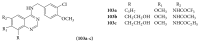
References
-
- Connolly D. J., Cusack D., O’Sullivan T. P., Guiry P. J. Synthesis of quinazolinones and quinazolines. Tetrahedron. 2005;61(43):10153–10202. doi: 10.1016/j.tet.2005.07.010. - DOI
-
- Abida, Nayyar P., Arpanarana M. An updated review: newer quinazoline derivatives under clinical trial. International Journal of Pharmaceutical & Biological Archive. 2011;2(6):1651–1657.
-
- Mhaske S. B., Argade N. P. The chemistry of recently isolated naturally occurring quinazolinone alkaloids. Tetrahedron. 2006;62(42):9787–9826. doi: 10.1016/j.tet.2006.07.098. - DOI
-
- Mahato A. K., Srivastava B., Nithya S. Chemistry structure activity relationship and biological activity of quinazoline-4(3H)-one derivatives. Inventi Rapid: MedChem. 2011;2(1)
-
- Armarego W. L. F. A Text Book of Quinazolines. 1963. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

