Levetiracetam Ameliorates L-DOPA-Induced Dyskinesia in Hemiparkinsonian Rats Inducing Critical Molecular Changes in the Striatum
- PMID: 25692070
- PMCID: PMC4322303
- DOI: 10.1155/2015/253878
Levetiracetam Ameliorates L-DOPA-Induced Dyskinesia in Hemiparkinsonian Rats Inducing Critical Molecular Changes in the Striatum
Abstract
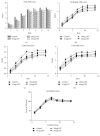
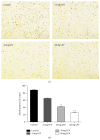
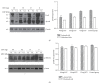
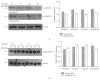
L-DOPA-induced dyskinesias (LID) remain a major problem of long-term therapy of Parkinson's disease. Levetiracetam, a new antiepileptic drug, has been shown to reduce LID, but the mechanisms underlying its effects are unknown. In this study, we assessed the effect of levetiracetam on key mediators of LID in rats with 6-hydroxydopamine (6-OHDA) lesions. Following chronic administration of L-DOPA (12 mg/kg, twice daily for 14 days), rats developed abnormal involuntary movements (AIMs), but co-administration of levetiracetam (15, 30, and 60 mg/kg) with equivalent L-DOPA dosing significantly reduced AIMs scores in a dose dependent manner. The effects of levetiracetam were associated with changes in striatal expression of ΔFosB, phosphorylated extracellular signal-regulated kinases 1 and 2 (p-ERK1/2), and phosphorylated cAMP-regulated phosphoprotein of 32 kDa (p-DARPP-32). These data support that levetiracetam acts at multiple sites in the pathogenetic cascade of LID, and that further understanding of these actions of antiepileptics may contribute to developing new LID therapies.
Figures
References
-
- Obeso J. A., Olanow C. W., Nutt J. G. Levodopa motor complications in Parkinson's disease. Trends in Neurosciences. 2000;23(10):S2–S7. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

