Cerebral amyloid angiopathy: emerging concepts
- PMID: 25692104
- PMCID: PMC4325636
- DOI: 10.5853/jos.2015.17.1.17
Cerebral amyloid angiopathy: emerging concepts
Abstract
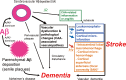
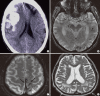
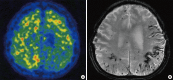
Cerebral amyloid angiopathy (CAA) involves cerebrovascular amyloid deposition and is classified into several types according to the amyloid protein involved. Of these, sporadic amyloid β-protein (Aβ)-type CAA is most commonly found in older individuals and in patients with Alzheimer's disease (AD). Cerebrovascular Aβ deposits accompany functional and pathological changes in cerebral blood vessels (CAA-associated vasculopathies). CAA-associated vasculopathies lead to development of hemorrhagic lesions [lobar intracerebral macrohemorrhage, cortical microhemorrhage, and cortical superficial siderosis (cSS)/focal convexity subarachnoid hemorrhage (SAH)], ischemic lesions (cortical infarction and ischemic changes of the white matter), and encephalopathies that include subacute leukoencephalopathy caused by CAA-associated inflammation/angiitis. Thus, CAA is related to dementia, stroke, and encephalopathies. Recent advances in diagnostic procedures, particularly neuroimaging, have enabled us to establish a clinical diagnosis of CAA without brain biopsies. Sensitive magnetic resonance imaging (MRI) methods, such as gradient-echo T2(*) imaging and susceptibility-weighted imaging, are useful for detecting cortical microhemorrhages and cSS. Amyloid imaging with amyloid-binding positron emission tomography (PET) ligands, such as Pittsburgh Compound B, can detect CAA, although they cannot discriminate vascular from parenchymal amyloid deposits. In addition, cerebrospinal fluid markers may be useful, including levels of Aβ40 for CAA and anti-Aβ antibody for CAA-related inflammation. Moreover, cSS is closely associated with transient focal neurological episodes (TFNE). CAA-related inflammation/angiitis shares pathophysiology with amyloid-related imaging abnormalities (ARIA) induced by Aβ immunotherapies in AD patients. This article reviews CAA and CAA-related disorders with respect to their epidemiology, pathology, pathophysiology, clinical features, biomarkers, diagnosis, treatment, risk factors, and future perspectives.
Keywords: Amyloid β-protein; Cerebral amyloid angiopathy; Cerebrospinal fluid; Cerebrovascular disorders; MRI; PET.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Scholz W. Studien zur Pathologie der Hirngefäße II. Die drusige Entartung der Hirnarterien und Kapillaren. Z ges Neurol Psychiat. 1938;162:694–715.
-
- Surbek B. L'angiopathie dyshorique (Morel) de l'écorce cérébrale. Acta Neuropathol. 1961;1:168–197.
-
- Pantelakis S. Un type particulier d'angiopathie sénile du système nerveux central: l'angiopathice congophile. Topographie et fréquence. Mschr Psychiatr Neurol. 1954;128:219–256. - PubMed
-
- Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18:311–324. - PubMed
-
- Yamada M, Naiki H. Cerebral amyloid angiopathy. Prog Mol Biol Transl Sci. 2012;51:41–78. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

