Dendritic cells induce Th2-mediated airway inflammatory responses to house dust mite via DNA-dependent protein kinase
- PMID: 25692509
- PMCID: PMC4333735
- DOI: 10.1038/ncomms7224
Dendritic cells induce Th2-mediated airway inflammatory responses to house dust mite via DNA-dependent protein kinase
Abstract
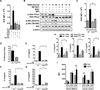
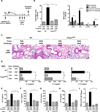
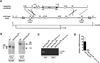
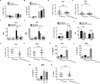
DNA-dependent protein kinase (DNA-PK) mediates double-stranded DNA break repair, V(D)J recombination and immunoglobulin class switch recombination, as well as innate immune and pro-inflammatory responses. However, there is limited information regarding the role of DNA-PK in adaptive immunity mediated by dendritic cells (DCs), which are the primary antigen-presenting cells in allergic asthma. Here we show that house dust mite induces DNA-PK phosphorylation, which is a marker of DNA-PK activation, in DCs via the generation of intracellular reactive oxygen species. We also demonstrate that pharmacological inhibition of DNA-PK, as well as the specific deletion of DNA-PK in DCs, attenuates the induction of allergic sensitization and Th2 immunity via a mechanism that involves the impaired presentation of mite antigens. Furthermore, pharmacological inhibition of DNA-PK following antigen priming similarly reduces the manifestations of mite-induced airway disease. Collectively, these findings suggest that DNA-PK may be a potential target for treatment of allergic asthma.
Figures





Similar articles
-
Dimethyl fumarate abrogates dust mite-induced allergic asthma by altering dendritic cell function.Immun Inflamm Dis. 2019 Sep;7(3):201-213. doi: 10.1002/iid3.262. Epub 2019 Jul 2. Immun Inflamm Dis. 2019. PMID: 31264384 Free PMC article.
-
The very low density lipoprotein receptor attenuates house dust mite-induced airway inflammation by suppressing dendritic cell-mediated adaptive immune responses.J Immunol. 2014 May 15;192(10):4497-509. doi: 10.4049/jimmunol.1301234. Epub 2014 Apr 14. J Immunol. 2014. PMID: 24733846 Free PMC article. Clinical Trial.
-
Notch signaling in T cells is essential for allergic airway inflammation, but expression of the Notch ligands Jagged 1 and Jagged 2 on dendritic cells is dispensable.J Allergy Clin Immunol. 2017 Oct;140(4):1079-1089. doi: 10.1016/j.jaci.2016.11.046. Epub 2017 Jan 19. J Allergy Clin Immunol. 2017. PMID: 28111308
-
Low-density lipoprotein receptor-related protein 1 attenuates house dust mite-induced eosinophilic airway inflammation by suppressing dendritic cell-mediated adaptive immune responses.J Allergy Clin Immunol. 2018 Oct;142(4):1066-1079.e6. doi: 10.1016/j.jaci.2017.10.044. Epub 2017 Dec 21. J Allergy Clin Immunol. 2018. PMID: 29274414 Free PMC article.
-
DNA-dependent protein kinase inhibition blocks asthma in mice and modulates human endothelial and CD4⁺ T-cell function without causing severe combined immunodeficiency.J Allergy Clin Immunol. 2015 Feb;135(2):425-40. doi: 10.1016/j.jaci.2014.09.005. Epub 2014 Oct 19. J Allergy Clin Immunol. 2015. PMID: 25441643 Free PMC article.
Cited by
-
TET2 Regulates 5-Hydroxymethylcytosine Signature and CD4+ T-Cell Balance in Allergic Rhinitis.Allergy Asthma Immunol Res. 2022 Mar;14(2):254-272. doi: 10.4168/aair.2022.14.2.254. Allergy Asthma Immunol Res. 2022. PMID: 35255541 Free PMC article.
-
Metabolic Plasticity in Dendritic Cell Responses: Implications in Allergic Asthma.J Immunol Res. 2017;2017:5134760. doi: 10.1155/2017/5134760. Epub 2017 Dec 14. J Immunol Res. 2017. PMID: 29387732 Free PMC article. Review.
-
Short palate, lung, and nasal epithelial clone 1 (SPLUNC1) level determines steroid-resistant airway inflammation in aging.Am J Physiol Lung Cell Mol Physiol. 2022 Jan 1;322(1):L102-L115. doi: 10.1152/ajplung.00315.2021. Epub 2021 Dec 1. Am J Physiol Lung Cell Mol Physiol. 2022. PMID: 34851736 Free PMC article.
-
Irg1/itaconate metabolic pathway is a crucial determinant of dendritic cells immune-priming function and contributes to resolute allergen-induced airway inflammation.Mucosal Immunol. 2022 Feb;15(2):301-313. doi: 10.1038/s41385-021-00462-y. Epub 2021 Oct 20. Mucosal Immunol. 2022. PMID: 34671116 Free PMC article.
-
Secrets of DNA-PKcs beyond DNA repair.NPJ Precis Oncol. 2024 Jul 23;8(1):154. doi: 10.1038/s41698-024-00655-1. NPJ Precis Oncol. 2024. PMID: 39043779 Free PMC article. Review.
References
-
- Kong X, Shen Y, Jiang N, Fei X, Mi J. Emerging roles of DNA-PK besides DNA repair. Cell Signal. 2011;23:1273–1280. - PubMed
-
- Meek K, Dang V, Lees-Miller SP. DNA-PK: the means to justify the ends? Adv Immunol. 2008;99:33–58. - PubMed
-
- Chen BP, Li M, Asaithamby A. New insights into the roles of ATM and DNA-PKcs in the cellular response to oxidative stress. Cancer Lett. 2012;327:103–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

