The Efficacy and Safety of Evekeo, Racemic Amphetamine Sulfate, for Treatment of Attention-Deficit/Hyperactivity Disorder Symptoms: A Multicenter, Dose-Optimized, Double-Blind, Randomized, Placebo-Controlled Crossover Laboratory Classroom Study
- PMID: 25692608
- PMCID: PMC4491157
- DOI: 10.1089/cap.2014.0176
The Efficacy and Safety of Evekeo, Racemic Amphetamine Sulfate, for Treatment of Attention-Deficit/Hyperactivity Disorder Symptoms: A Multicenter, Dose-Optimized, Double-Blind, Randomized, Placebo-Controlled Crossover Laboratory Classroom Study
Abstract
Objective: The study goal was to determine the efficacy and safety of an optimal dose of Evekeo, racemic amphetamine sulfate, 1:1 d-amphetamine and l-amphetamine (R-AMPH), compared to placebo in treating children with attention-deficit/hyperactivity disorder (ADHD) in a laboratory classroom setting.
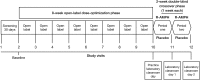
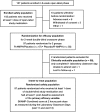
Methods: A total of 107 children ages 6-12 years were enrolled in this multicenter, dose-optimized, randomized, double-blind, placebo-controlled crossover study. After 8 weeks of open-label dose optimization, 97 subjects were randomized to 2 weeks of double-blind treatment in the sequence of R-AMPH followed by placebo (n=47) or placebo followed by R-AMPH (n=50). Efficacy measures included the Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) Rating Scale and Permanent Product Measure of Performance (PERMP) administered predose and at 0.75, 2, 4, 6, 8, and 10 hours postdose on 2 laboratory classroom days. Safety assessments included physical examination, chemistry, hematology, vital signs, and treatment-emergent adverse events (TEAEs).
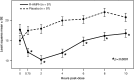
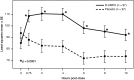
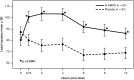
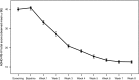
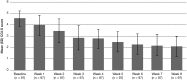
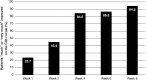
Results: Compared to placebo, a single daily dose of R-AMPH significantly improved SKAMP-Combined scores (p<0.0001) at each time point tested throughout the laboratory classroom days, with effect onset 45 minutes postdose and extending through 10 hours. R-AMPH significantly improved PERMP number of problems attempted and correct (p<0.0001) throughout the laboratory classroom days. During the twice-daily dose-optimization open-label phase, improvements were observed with R-AMPH in scores of the ADHD-Rating Scale IV and Clinical Global Impressions Severity and Improvement Scales. TEAEs and changes in vital signs associated with R-AMPH were generally mild and not unexpected. The most common TEAEs in the open-label phase were decreased appetite (27.6%), upper abdominal pain (14.3%), irritability (14.3%), and headache (13.3%).
Conclusions: Compared to placebo, R-AMPH was effective in treating children aged 6-12 years with ADHD, beginning at 45 minutes and continuing through 10 hours postdose, and was well tolerated.
Trial registration: ClinicalTrials.gov identifier: NCT01986062. https://clinicaltrials.gov/ct2/show/NCT01986062.
Figures
References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed., Text Revision (DSM-IV-TR). Washington, DC: American Psychiatric Association; 2000
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. (DSM-5). Arlington, VA: American Psychiatric Association; 2013
-
- Arnold LE, Wender PH, McCloskey K, Snyder SH: Levoamphetamine and dextroamphetamine: Comparative efficacy in the hyperkinetic syndrome. Assessment by target symptoms. Arch Gen Psychiatry 27:816–822, 1972 - PubMed
-
- Barbaresi WJ, Katusic SK, Colligan RC, Weaver AL, Jacobsen SJ: Modifiers of long-term school outcomes for children with attention-deficit/hyperactivity disorder: Does treatment with stimulant medication make a difference? Results from a population-based study. J Dev Behav Pediatr 28:274–287, 2007 - PubMed
-
- Biederman J, Lopez FA, Boellner SW, Chandler MC: A randomized, double-blind, placebo-controlled, parallel-group study of SLI381 (Adderall XR) in children with attention-deficit/hyperactivity disorder. Pediatrics 110:258–266, 2002 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
