Differential mRNA expression and glucocorticoid-mediated regulation of TRPM6 and TRPM7 in the heart and kidney throughout murine pregnancy and development
- PMID: 25692682
- PMCID: PMC4333289
- DOI: 10.1371/journal.pone.0117978
Differential mRNA expression and glucocorticoid-mediated regulation of TRPM6 and TRPM7 in the heart and kidney throughout murine pregnancy and development
Abstract
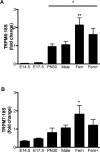
The transient receptor potential (TRP) channels TRPM6 and TRPM7 are critically involved in maintaining whole body and cellular Mg2+ homeostasis and ensuring the normal function of organs such as the heart and kidney. However, we do not know how the expression of TRPM6 and TPRM7 in these organs changes throughout fetal development and adult life, and whether this expression can be hormonally regulated. This study determined the ontogeny of TRPM6 and TRPM7 mRNA expression from mid-gestation through to adulthood in the mouse. In a second series of experiments, we examined how maternal administration of the glucocorticoids corticosterone and dexamethasone between embryonic days 12.5-15 affected TRPM6 and TRPM7 channel mRNA expression in the mother and fetus. Whilst renal TRPM7 expression was relatively constant throughout development, renal TRPM6 expression was markedly upregulated after birth. In contrast, cardiac TRPM7 expression was 2-4 fold higher in the fetus than in the adult. Surprisingly, TRPM6 expression was detected in the fetal heart (qPCR and in situ hybridization). Glucocorticoid administration during gestation increased fetal cardiac expression of both channels without affecting renal expression. In contrast, in the dam renal TRPM6 and TRPM7 expression was increased by glucocorticoids with no change in the cardiac channel expression. These data suggest that TRPM6 and TRPM7 channels are important in organogenesis, and that elevated maternal glucocorticoid levels can alter the expression of these channels. This suggests that perturbations in hormonal regulatory systems during pregnancy may adversely impact upon normal fetal development, at least in part by altering expression of TRPM channels.
Conflict of interest statement
Figures






References
-
- Wolf FI, Trapani V (2008) Cell (patho)physiology of magnesium. Clinical science 114: 27–35. - PubMed
-
- Konrad M, Schlingmann KP, Gudermann T (2004) Insights into the molecular nature of magnesium homeostasis. American journal of physiology Renal physiology 286: F599–F605. - PubMed
-
- Schlingmann K, Weber S, Peters M, Niemann Nejsum L, Vitzthum H, et al. (2002) Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nature genetics 31: 166–170. - PubMed
-
- Walder RY, Landau D, Meyer P, Shalev H, Tsolia M, et al. (2002) Mutation of TRPM6 causes familial hypomagnesemia with secondary hypocalcemia. Nature genetics 31: 171–174. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

