Comparative study on the immunogenicity and safety of a purified chick embryo cell rabies vaccine (PCECV) administered according to two different simulated post exposure intramuscular regimens (Zagreb versus Essen)
- PMID: 25692792
- PMCID: PMC4514298
- DOI: 10.4161/21645515.2014.995059
Comparative study on the immunogenicity and safety of a purified chick embryo cell rabies vaccine (PCECV) administered according to two different simulated post exposure intramuscular regimens (Zagreb versus Essen)
Abstract
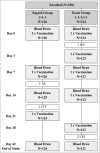
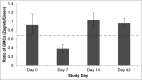
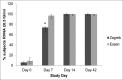
Despite availability of effective rabies vaccines, India has the highest global mortality rate for rabies. Low socio-economic communities are most affected due to lack of awareness of the disease and poor compliance to post-exposure prophylactic regimens. Currently, the only approved intramuscular regimen for post-exposure prophylaxis (PEP) against rabies in India is the Essen regimen, which consists of 5 injections administered over 5 separate days in a period of one month. The high number of doses and clinical visits, however, are major reasons for non-compliance, and thus a shorter regimen would be beneficial. In a simulated PEP trial in healthy, adult subjects, this study evaluated whether purified chick embryo cell vaccine (PCECV), administered according to the WHO-recommended 4-dose/3 visit Zagreb vaccination regimen is of equal immunogenicity and safety as the standard Essen regimen in Indian subjects. Two hundred and 50 healthy adults were enrolled and randomized into a Zagreb or Essen group, each receiving PCECV according to their respective regimen. Blood samples were collected on Days 0, 7, 14 and 42 and analyzed using the rapid fluorescent focus inhibition test (RFFIT). By Day 14, all subjects across both groups attained rabies virus neutralizing antibody (RVNA) concentrations of ≥ 0.5IU/ml. The Zagreb regimen was then demonstrated to be immunologically non-inferior to the Essen regimen by Day 14, which was the primary endpoint of the study. No safety issues were noted and the occurrence of adverse events was similar in both groups (17% and 15%, respectively). NCT01365494. CTRI No.: CTRI/2011/07/001857.
Keywords: AE, adverse event; Essen; GMC, geometric mean concentration; IM, intramuscular; India; PCECV; PCECV, purified chick embryo cell rabies vaccine; PEP, post-exposure prophylaxis; RFFIT, rapid fluorescent focus inhibition test; RVNA, rabies virus neutralizing antibody; SAE, serious adverse event; Zagreb; rabies.
Figures
References
-
- (WHO) WHO . Rabies Vaccines: WHO position paper. Weekly epidemiological record 2010; 32:309-20; PMID:18064757 - PubMed
-
- Menezes R. Rabies in India. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne 2008; 178:564-6; PMID:18299543; http://dx.doi.org/10.1503/cmaj.071488 - DOI - PMC - PubMed
-
- Hemachudha T, Wacharapluesadee S, Mitrabhakdi E, Wilde H, Morimoto K, Lewis RA. Pathophysiology of human paralytic rabies. Journal of neurovirology 2005; 11:93-100; PMID:15804967; http://dx.doi.org/10.1080/13550280590900409 - DOI - PubMed
-
- Gadre G, Satishchandra P, Mahadevan A, Suja MS, Madhusudana SN, Sundaram C, Shankar SK. Rabies viral encephalitis: clinical determinants in diagnosis with special reference to paralytic form. Journal of neurology, neurosurgery, and psychiatry 2010; 81:812-20; PMID:19965838; http://dx.doi.org/10.1136/jnnp.2009.185504 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
