Glucosidase II and MRH-domain containing proteins in the secretory pathway
- PMID: 25692846
- PMCID: PMC4411176
- DOI: 10.2174/1389203716666150213160438
Glucosidase II and MRH-domain containing proteins in the secretory pathway
Abstract
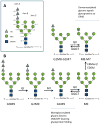
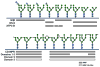
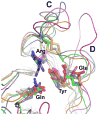
N-glycosylation in the endoplasmic reticulum (ER) consists of the transfer of a preassembled glycan conserved among species (Glc3Man9GlcNAc2) from a lipid donor to a consensus sequence within a nascent protein that is entering the ER. The protein-linked glycans are then processed by glycosidases and glycosyltransferases in the ER producing specific structures that serve as signalling molecules for the fate of the folding glycoprotein: to stay in the ER during the folding process, to be retrotranslocated to the cytosol for proteasomal degradation if irreversibly misfolded, or to pursue transit through the secretory pathway as a mature glycoprotein. In the ER, each glycan signalling structure is recognized by a specific lectin. A domain similar to that of the mannose 6-phosphate receptors (MPRs) has been identified in several proteins of the secretory pathway. These include the beta subunit of glucosidase II (GII), a key enzyme in the early processing of the transferred glycan that removes middle and innermost glucoses and is involved in quality control of glycoprotein folding in the ER (QC), the lectins OS-9 and XTP3-B, proteins involved in the delivery of ER misfolded proteins to degradation (ERAD), the gamma subunit of the Golgi GlcNAc-1-phosphotransferase, an enzyme involved in generating the mannose 6-phosphate (M6P) signal for sorting acidic hydrolases to lysosomes, and finally the MPRs that deliver those hydrolytic enzymes to the lysosome. Each of the MRH-containing proteins recognizes a different signalling N-glycan structure. Three-dimensional structures of some of the MRH domains have been solved, providing the basis to understand recognition mechanisms.
Conflict of interest statement
Figures



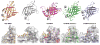
References
-
- Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annual Review of Biochemistry. 1985;54:631–64. - PubMed
-
- Aebi M, Bernasconi R, Clerc S, Molinari M. N-glycan structures: Recognition and processing in the er. Trends Biochem Sci. 2010;35(2):74–82. - PubMed
-
- Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the swiss-prot database. Biochim Biophys Acta. 1999;1473(1):4–8. - PubMed
-
- Nilsson IM, von Heijne G. Determination of the distance between the oligosaccharyltransferase active site and the endoplasmic reticulum membrane. J Biol Chem. 1993;268(8):5798–801. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

