A cell-permeable fluorescent polymeric thermometer for intracellular temperature mapping in mammalian cell lines
- PMID: 25692871
- PMCID: PMC4333297
- DOI: 10.1371/journal.pone.0117677
A cell-permeable fluorescent polymeric thermometer for intracellular temperature mapping in mammalian cell lines
Abstract
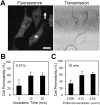
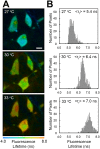
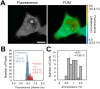
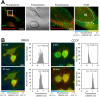
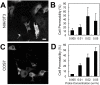
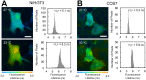
Changes in intracellular temperatures reflect the activity of the cell. Thus, the tool to measure intracellular temperatures could provide valuable information about cellular status. We previously reported a method to analyze the intracellular temperature distribution using a fluorescent polymeric thermometer (FPT) in combination with fluorescence lifetime imaging microscopy (FLIM). Intracellular delivery of the FPT used in the previous study required microinjection. We now report a novel FPT that is cell permeable and highly photostable, and we describe the application of this FPT to the imaging of intracellular temperature distributions in various types of mammalian cell lines. This cell-permeable FPT displayed a temperature resolution of 0.05°C to 0.54°C within the range from 28°C to 38°C in HeLa cell extracts. Using our optimized protocol, this cell-permeable FPT spontaneously diffused into HeLa cells within 10 min of incubation and exhibited minimal toxicity over several hours of observation. FLIM analysis confirmed a temperature difference between the nucleus and the cytoplasm and heat production near the mitochondria, which were also detected previously using the microinjected FPT. We also showed that this cell-permeable FPT protocol can be applied to other mammalian cell lines, COS7 and NIH/3T3 cells. Thus, this cell-permeable FPT represents a promising tool to study cellular states and functions with respect to temperature.
Conflict of interest statement
Figures


References
-
- Somero GN (1995) Proteins and temperature. Annu Rev Physiol 57: 43–68. - PubMed
-
- Inada N, Uchiyama S (2013) Methods and benefits of imaging the temperature distribution inside living cells. Imaging Med 5: 303–305.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

