Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer
- PMID: 25693012
- PMCID: PMC5584549
- DOI: 10.1056/NEJMoa1413513
Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer
Abstract
Background: In patients with metastatic breast cancer that is positive for human epidermal growth factor receptor 2 (HER2), progression-free survival was significantly improved after first-line therapy with pertuzumab, trastuzumab, and docetaxel, as compared with placebo, trastuzumab, and docetaxel. Overall survival was significantly improved with pertuzumab in an interim analysis without the median being reached. We report final prespecified overall survival results with a median follow-up of 50 months.
Methods: We randomly assigned patients with metastatic breast cancer who had not received previous chemotherapy or anti-HER2 therapy for their metastatic disease to receive the pertuzumab combination or the placebo combination. The secondary end points of overall survival, investigator-assessed progression-free survival, independently assessed duration of response, and safety are reported. Sensitivity analyses were adjusted for patients who crossed over from placebo to pertuzumab after the interim analysis.
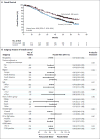
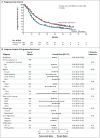
Results: The median overall survival was 56.5 months (95% confidence interval [CI], 49.3 to not reached) in the group receiving the pertuzumab combination, as compared with 40.8 months (95% CI, 35.8 to 48.3) in the group receiving the placebo combination (hazard ratio favoring the pertuzumab group, 0.68; 95% CI, 0.56 to 0.84; P<0.001), a difference of 15.7 months. This analysis was not adjusted for crossover to the pertuzumab group and is therefore conservative. Results of sensitivity analyses after adjustment for crossover were consistent. Median progression-free survival as assessed by investigators improved by 6.3 months in the pertuzumab group (hazard ratio, 0.68; 95% CI, 0.58 to 0.80). Pertuzumab extended the median duration of response by 7.7 months, as independently assessed. Most adverse events occurred during the administration of docetaxel in the two groups, with long-term cardiac safety maintained.
Conclusions: In patients with HER2-positive metastatic breast cancer, the addition of pertuzumab to trastuzumab and docetaxel, as compared with the addition of placebo, significantly improved the median overall survival to 56.5 months and extended the results of previous analyses showing the efficacy of this drug combination. (Funded by F. Hoffmann-La Roche and Genentech; CLEOPATRA ClinicalTrials.gov number, NCT00567190.).
Figures
Comment in
-
Breast cancer: CLEOPATRA sheds light on how to tackle metastatic disease.Nat Rev Clin Oncol. 2015 Apr;12(4):188. doi: 10.1038/nrclinonc.2015.40. Epub 2015 Mar 3. Nat Rev Clin Oncol. 2015. PMID: 25734635 No abstract available.
-
Treatment of HER2-positive metastatic breast cancer.N Engl J Med. 2015 May 14;372(20):1964-5. doi: 10.1056/NEJMc1503446. N Engl J Med. 2015. PMID: 25970056 Free PMC article. No abstract available.
-
Treatment of HER2-positive metastatic breast cancer.N Engl J Med. 2015 May 14;372(20):1964. doi: 10.1056/NEJMc1503446. N Engl J Med. 2015. PMID: 25970057 No abstract available.
References
-
- Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–68. - PubMed
-
- Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res. 2004;64:2343–6. - PubMed
-
- Scheuer W, Friess T, Burtscher H, Bossenmaier B, Endl J, Hasmann M. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 2009;69:9330–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
