Direct binding of retromer to human papillomavirus type 16 minor capsid protein L2 mediates endosome exit during viral infection
- PMID: 25693203
- PMCID: PMC4334968
- DOI: 10.1371/journal.ppat.1004699
Direct binding of retromer to human papillomavirus type 16 minor capsid protein L2 mediates endosome exit during viral infection
Abstract
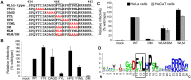
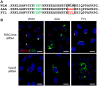
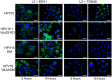
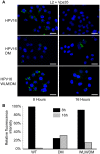
Trafficking of human papillomaviruses to the Golgi apparatus during virus entry requires retromer, an endosomal coat protein complex that mediates the vesicular transport of cellular transmembrane proteins from the endosome to the Golgi apparatus or the plasma membrane. Here we show that the HPV16 L2 minor capsid protein is a retromer cargo, even though L2 is not a transmembrane protein. We show that direct binding of retromer to a conserved sequence in the carboxy-terminus of L2 is required for exit of L2 from the early endosome and delivery to the trans-Golgi network during virus entry. This binding site is different from known retromer binding motifs and can be replaced by a sorting signal from a cellular retromer cargo. Thus, HPV16 is an unconventional particulate retromer cargo, and retromer binding initiates retrograde transport of viral components from the endosome to the trans-Golgi network during virus entry. We propose that the carboxy-terminal segment of L2 protein protrudes through the endosomal membrane and is accessed by retromer in the cytoplasm.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Parkin DM, Bray F (2006) Chapter 2: The burden of HPV-related cancers. Vaccine 24 Suppl 3: S3/11–25. - PubMed
-
- Liu WJ, Gissmann L, Sun XY, Kanjanahaluethai A, Muller M, et al. (1997) Sequence close to the N-terminus of L2 protein is displayed on the surface of bovine papillomavirus type 1 virions. Virology 227: 474–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

