Randomised clinical trial: the 5-HT4 agonist revexepride in patients with gastro-oesophageal reflux disease who have persistent symptoms despite PPI therapy
- PMID: 25693609
- PMCID: PMC5024018
- DOI: 10.1111/apt.13115
Randomised clinical trial: the 5-HT4 agonist revexepride in patients with gastro-oesophageal reflux disease who have persistent symptoms despite PPI therapy
Abstract
Background: A substantial proportion of patients with gastro-oesophageal reflux disease (GERD) have only a partial response to proton pump inhibitor (PPI) therapy. Prokinetic drugs may improve reflux symptoms by enhancing oesophageal motility and gastric emptying.
Aim: To evaluate the effect of revexepride, a novel prokinetic 5-hydroxytryptamine type 4 (5-HT4 ) receptor agonist, compared with placebo, in patients with GERD who have a partial response to PPIs.
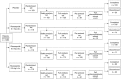
Methods: A phase 2b, double-blind, parallel-group study was conducted, in which patients were randomised to one of three revexepride treatment groups (0.1, 0.5 and 2.0 mg three times daily) or placebo (1:1:1:1 ratio). Daily e-diary data captured patients' symptoms over an 8-week treatment period. The primary efficacy outcome was the weekly percentage of regurgitation-free days in the second half of the study (weeks 5-8).
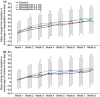
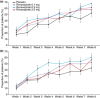
Results: In total, 480 patients were randomised and 477 received treatment (mean age 47.9 years; 61% women). The mean percentage of regurgitation-free days increased from baseline (range, 15.0-18.8%) to week 8 (62.3-70.5%) in all four study arms; however, there were no statistically significant differences in this change between placebo and the three treatment arms. No dose-dependent relationship in treatment effect was observed for any of the study endpoints. The incidence of treatment-emergent adverse events (TEAEs) was revexepride dose-dependent. Only one serious TEAE occurred and none resulted in death.
Conclusions: Revexepride was no more effective than placebo in controlling regurgitation in patients with GERD symptoms partially responsive to PPIs. Revexepride was well tolerated. ClinicalTrials.gov Identifier: NCT01472939.
© 2015 Shire Development LLC. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures

Similar articles
-
Randomized clinical trial: effect of the 5-HT4 receptor agonist revexepride on reflux parameters in patients with persistent reflux symptoms despite PPI treatment.Neurogastroenterol Motil. 2015 Feb;27(2):258-68. doi: 10.1111/nmo.12484. Epub 2014 Dec 21. Neurogastroenterol Motil. 2015. PMID: 25530111 Free PMC article. Clinical Trial.
-
Randomized clinical trial: a controlled pilot trial of the 5-HT4 receptor agonist revexepride in patients with symptoms suggestive of gastroparesis.Neurogastroenterol Motil. 2016 Apr;28(4):487-97. doi: 10.1111/nmo.12736. Neurogastroenterol Motil. 2016. PMID: 27010235 Clinical Trial.
-
A phase 1 randomized study evaluating the effect of omeprazole on the pharmacokinetics of a novel 5-hydroxytryptamine receptor 4 agonist, revexepride (SSP-002358), in healthy adults.Drug Des Devel Ther. 2015 Feb 27;9:1257-68. doi: 10.2147/DDDT.S64621. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 25767373 Free PMC article. Clinical Trial.
-
Review article: physiologic and clinical effects of proton pump inhibitors on non-acidic and acidic gastro-oesophageal reflux.Aliment Pharmacol Ther. 2006 Mar;23 Suppl 1:25-32. doi: 10.1111/j.1365-2036.2006.02802.x. Aliment Pharmacol Ther. 2006. PMID: 16483267 Review.
-
Randomised trials of proton pump inhibitors for gastro-oesophageal reflux disease in patients with asthma: an updated systematic review and meta-analysis.BMJ Open. 2021 Aug 10;11(8):e043860. doi: 10.1136/bmjopen-2020-043860. BMJ Open. 2021. PMID: 34376437 Free PMC article.
Cited by
-
Pharmacokinetics, absorption, and excretion of radiolabeled revexepride: a Phase I clinical trial using a microtracer and accelerator mass spectrometry-based approach.Drug Des Devel Ther. 2016 Sep 27;10:3125-3132. doi: 10.2147/DDDT.S107843. eCollection 2016. Drug Des Devel Ther. 2016. PMID: 27729771 Free PMC article. Clinical Trial.
-
A Systematic Review and Meta-analysis of Randomized Control Trials: Combination Treatment With Proton Pump Inhibitor Plus Prokinetic for Gastroesophageal Reflux Disease.J Neurogastroenterol Motil. 2021 Apr 30;27(2):165-175. doi: 10.5056/jnm20161. J Neurogastroenterol Motil. 2021. PMID: 33795539 Free PMC article.
-
Management of refractory typical GERD symptoms.Nat Rev Gastroenterol Hepatol. 2016 May;13(5):281-94. doi: 10.1038/nrgastro.2016.50. Epub 2016 Apr 14. Nat Rev Gastroenterol Hepatol. 2016. PMID: 27075264 Review.
-
Prokinetics in the Management of Functional Gastrointestinal Disorders.Curr Gastroenterol Rep. 2017 Sep 8;19(10):53. doi: 10.1007/s11894-017-0593-6. Curr Gastroenterol Rep. 2017. PMID: 28887755 Review.
-
Recent Advances in the Pharmacological Management of Gastroesophageal Reflux Disease.Dig Dis Sci. 2017 Dec;62(12):3298-3316. doi: 10.1007/s10620-017-4830-5. Epub 2017 Nov 6. Dig Dis Sci. 2017. PMID: 29110162 Review.
References
-
- Kulig M, Leodolter A, Vieth M, et al Quality of life in relation to symptoms in patients with gastro‐oesophageal reflux disease – an analysis based on the ProGERD initiative. Aliment Pharmacol Ther 2003; 18: 767–76. - PubMed
-
- Vakil N, Veldhuyzen van Zanten S, Kahrilas P, et al The Montreal definition and classification of gastro‐esophageal reflux disease (GERD) – a global evidence‐based consensus. Am J Gastroenterol 2006; 101: 1900–20. - PubMed
-
- Voutilainen M, Sipponen P, Mecklin JP, et al Gastroesophageal reflux disease: prevalence, clinical, endoscopic and histopathological findings in 1,128 consecutive patients referred for endoscopy due to dyspeptic and reflux symptoms. Digestion 2000; 61: 6–13. - PubMed
-
- Jaspersen D, Kulig M, Labenz J, et al Prevalence of extra‐oesophageal manifestations in gastro‐oesophageal reflux disease: an analysis based on the ProGERD Study. Aliment Pharmacol Ther 2003; 17: 1515–20. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
