Impact of the Mobile HealthPROMISE Platform on the Quality of Care and Quality of Life in Patients With Inflammatory Bowel Disease: Study Protocol of a Pragmatic Randomized Controlled Trial
- PMID: 25693610
- PMCID: PMC4376196
- DOI: 10.2196/resprot.4042
Impact of the Mobile HealthPROMISE Platform on the Quality of Care and Quality of Life in Patients With Inflammatory Bowel Disease: Study Protocol of a Pragmatic Randomized Controlled Trial
Abstract
Background: Inflammatory bowel disease (IBD) is a chronic condition of the bowel that affects over 1 million people in the United States. The recurring nature of disease makes IBD patients ideal candidates for patient-engaged care that is centered on enhanced self-management and improved doctor-patient communication. In IBD, optimal approaches to management vary for patients with different phenotypes and extent of disease and past surgical history. Hence, a single quality metric cannot define a heterogeneous disease such as IBD, unlike hypertension and diabetes. A more comprehensive assessment may be provided by complementing traditional quality metrics with measures of the patient's quality of life (QOL) through an application like HealthPROMISE.
Objective: The objective of this pragmatic randomized controlled trial is to determine the impact of the HealthPROMISE app in improving outcomes (quality of care [QOC], QOL, patient adherence, disease control, and resource utilization) as compared to a patient education app. Our hypothesis is that a patient-centric self-monitoring and collaborative decision support platform will lead to sustainable improvement in overall QOL for IBD patients.
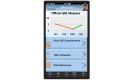
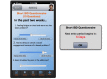
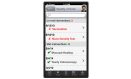
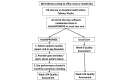
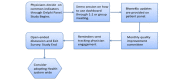
Methods: Participants will be recruited during face-to-face visits and randomized to either an interventional (ie, HealthPROMISE) or control (ie, education app). Patients in the HealthPROMISE arm will be able to update their information and receive disease summary, quality metrics, and a graph showing the trend of QOL (SIBDQ) scores and resource utilization over time. Providers will use the data for collaborative decision making and quality improvement interventions at the point of care. Patients in the control arm will enter data at baseline, during office visits, and at the end of the study but will not receive any decision support (trend of QOL, alert, or dashboard views).
Results: Enrollment in the trial will be starting in first quarter of 2015. It is intended that up to 300 patients with IBD will be recruited into the study (with 1:1 allocation ratio). The primary endpoint is number of quality indicators met in HealthPROMISE versus control arm. Secondary endpoints include decrease in number of emergency visits due to IBD, decrease in number of hospitalization due to IBD, change in generic QOL score from baseline, proportion of patients in each group who meet all eligible outpatient quality metrics, and proportion of patients in disease control in each group. In addition, we plan to conduct protocol analysis of intervention patients with adequate HealthPROMISE utilization (more than 6 log-ins with data entry from week 0 through week 52) achieving above mentioned primary and secondary endpoints.
Conclusions: HealthPROMISE is a unique cloud-based patient-reported outcome (PRO) and decision support tool that empowers both patients and providers. Patients track their QOL and symptoms, and providers can use the visual data in real time (integrated with electronic health records [EHRs]) to provide better care to their entire patient population. Using pragmatic trial design, we hope to show that IBD patients who participate in their own care and share in decision making have appreciably improved outcomes when compared to patients who do not.
Trial registration: ClinicalTrials.gov NCT02322307; https://clinicaltrials.gov/ct2/show/NCT02322307 (Archived by WebCite at http://www.webcitation.org/6W8PoYThr).
Keywords: engagement; mHealth; medical informatics; patient reported outcome.
Conflict of interest statement
Conflicts of Interest: The app was developed in-house at Sinai AppLab.
Figures
References
-
- Cohen RD. The quality of life in patients with Crohn's disease. Aliment Pharmacol Ther. 2002 Sep;16(9):1603–1609. http://onlinelibrary.wiley.com/resolve/openurl?genre=article&sid=nlm:pub... - PubMed
-
- Irvine EJ. Quality of life issues in patients with inflammatory bowel disease. Am J Gastroenterol. 1997 Dec;92(12 Suppl):18S–24S. - PubMed
-
- Atreja A, Bahuva R, Achkar JP, Brzezinski A, Shen B, Kandiel A, Lashner B. Can patients reliably report quality indicators and disease phenotype? Proceedings of the 11th Advances in Inflammatory Bowel Diseases, Crohn’s & Colitis Foundation’s Clinical and Research Conference; 2011 Dec 1-3; Hollywood Florida. Advances in Inflammatory Bowel Diseases, Crohn's & Colitis Foundation's Clinical and Research Conference; 2011; Hollywood, FL. 2011. Dec,
-
- De Feo J, Barnard W. Juran Institute's Six Sigma Breakthrough and Beyond: Quality Performance Breakthrough Methods. New York: McGraw-Hill; 2004.
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous