Primaquine or other 8-aminoquinoline for reducing Plasmodium falciparum transmission
- PMID: 25693791
- PMCID: PMC4455224
- DOI: 10.1002/14651858.CD008152.pub4
Primaquine or other 8-aminoquinoline for reducing Plasmodium falciparum transmission
Update in
-
Primaquine or other 8-aminoquinolines for reducing Plasmodium falciparum transmission.Cochrane Database Syst Rev. 2018 Feb 2;2(2):CD008152. doi: 10.1002/14651858.CD008152.pub5. Cochrane Database Syst Rev. 2018. PMID: 29393511 Free PMC article.
Abstract
Background: Mosquitoes become infected with Plasmodium when they ingest gametocyte-stage parasites from an infected person's blood. Plasmodium falciparum gametocytes are sensitive to 8-aminoquinolines (8AQ), and consequently these drugs could prevent parasite transmission from infected people to mosquitoes and reduce the incidence of malaria. However, when used in this way, these drugs will not directly benefit the individual.In 2010, the World Health Organization (WHO) recommended a single dose of primaquine (PQ) at 0.75 mg/kg alongside treatment for P. falciparum malaria to reduce transmission in areas approaching malaria elimination. In 2013, the WHO revised this to 0.25 mg/kg to reduce risk of harms in people with G6PD deficiency.
Objectives: To assess the effects of PQ (or an alternative 8AQ) given alongside treatment for P. falciparum malaria on malaria transmission and on the occurrence of adverse events.
Search methods: We searched the following databases up to 5 January 2015: the Cochrane Infectious Diseases Group Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library (Issue 1, 2015); MEDLINE (1966 to 5 January 2015); EMBASE (1980 to 5 January 2015); LILACS (1982 to 5 January 2015); metaRegister of Controlled Trials (mRCT); and the WHO trials search portal using 'malaria*', 'falciparum', 'primaquine', 8-aminoquinoline and eight individual 8AQ drug names as search terms. In addition, we searched conference proceedings and reference lists of included studies, and contacted researchers and organizations.
Selection criteria: Randomized controlled trials (RCTs) or quasi-RCTs in children or adults, comparing PQ (or alternative 8AQ) as a single dose or short course alongside treatment for P. falciparum malaria, with the same malaria treatment given without PQ/8AQ.
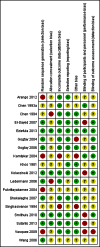
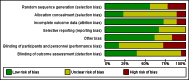
Data collection and analysis: Two review authors independently screened all abstracts, applied inclusion criteria and extracted data. We sought evidence of an impact on transmission (community incidence), infectiousness (mosquitoes infected from humans) and potential infectiousness (gametocyte measures). We calculated the area under the curve (AUC) for gametocyte density over time for comparisons for which data were available. We sought data on haematological and other adverse effects, asexual parasite clearance time and recrudescence. We stratified the analysis by artemisinin and non-artemisinin treatments; and by PQ dose (low < 0.4 mg/kg; medium ≥ 0.4 to < 0.6 mg/kg; high ≥ 0.6 mg/kg). We used the GRADE approach to assess evidence quality.
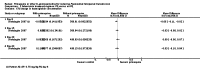
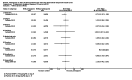
Main results: We included 17 RCTs and one quasi-RCT. Eight trials tested for G6PD status: six then excluded participants with G6PD deficiency, one included only those with G6PD deficiency, and one included all irrespective of status. The remaining 10 trials either did not report on whether they tested (eight trials), or reported that they did not test (two trials).Nine trials included study arms with artemisinin-based treatments and eleven included study arms with non-artemisinin-based treatments.Only one trial evaluated PQ given as a single dose of less than 0.4 mg/kg. PQ with artemisinin-based treatments: No trials evaluated effects on malaria transmission directly (incidence, prevalence or entomological inoculation rate) and none evaluated infectiousness to mosquitoes. For potential infectiousness, the proportion of people with detectable gametocytaemia on day eight was reduced by around two-thirds with the high dose PQ category (RR 0.29, 95% confidence interval (CI) 0.22 to 0.37; seven trials, 1380 participants, high quality evidence) and the medium dose PQ category (RR 0.30, 95% CI 0.16 to 0.56; one trial, 219 participants, moderate quality evidence). For the low dose category, the effect size was smaller and the 95% CIs include the possibility of no effect (dose: 0.1 mg/kg: RR 0.67, 95% CI 0.44 to 1.02; one trial, 223 participants, low quality evidence). Reductions in log(10)AUC estimates for gametocytaemia on days 1 to 43 with medium and high doses ranged from 24.3% to 87.5%. For haemolysis, one trial reported percent change in mean haemoglobin against baseline and did not detect a difference between the two arms (very low quality evidence). PQ with non-artemisinin treatments: No trials assessed effects on malaria transmission directly. Two small trials from the same laboratory in China evaluated infectiousness to mosquitoes, and reported that infectivity was eliminated on day 8 in 15/15 patients receiving high dose PQ compared to 1/15 in the control group (low quality evidence). For potential infectiousness, the proportion of people with detectable gametocytaemia on day 8 was reduced by three-fifths with high dose PQ category (RR 0.39, 95% CI 0.25 to 0.62; four trials, 186 participants, high quality evidence), and by around two-fifths with medium dose category (RR 0.60, 95% CI 0.49 to 0.75; one trial, 216 participants, high quality evidence), with no trial in the low dose PQ category reporting this outcome. Reduction in log(10)AUC for gametocytaemia days 1 to 43 were 24.3% and 27.1% for two arms in one trial giving medium dose PQ. No trials systematically sought evidence of haemolysis.Two trials evaluated the 8AQ bulaquine, and suggest the effects may be greater than PQ, but the small number of participants (N = 112) preclude a definite conclusion.
Authors' conclusions: In individual patients, PQ added to malaria treatments reduces gametocyte prevalence, but this is based on trials using doses of more than 0.4 mg/kg. Whether this translates into preventing people transmitting malaria to mosquitoes has rarely been tested in controlled trials, but there appeared to be a strong reduction in infectiousness in the two small studies that evaluated this. No included trials evaluated whether this policy has an impact on community malaria transmission.For the currently recommended low dose regimen, there is currently little direct evidence to be confident that the effect of reduction in gametocyte prevalence is preserved, or that it is safe in people with G6PD deficiency.
Figures




















Update of
-
Primaquine or other 8-aminoquinoline for reducing P. falciparum transmission.Cochrane Database Syst Rev. 2014 Jun 30;(6):CD008152. doi: 10.1002/14651858.CD008152.pub3. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2015 Feb 19;(2):CD008152. doi: 10.1002/14651858.CD008152.pub4. PMID: 24979199 Free PMC article. Updated.
References
-
- Arango EA, Upegui UA, Carmona-Fonseca J. Efficacy of different primaquine-based antimalarial regimens againstPlasmodium falciparum gametocytemia. Acta Tropica. 2012;122((2012)):177–82. - PubMed
-
- Chen PQ, Li GQ, Guo XB, Fu YX, He KR, Fu LC, et al. A double blind study on the infectivity of gametocytes of P. falciparum in patients treated with mefloquine and Fansimef. Journal of Guangzhou College of Traditional Chinese Medicine. 1993;10((1)):1–5.
-
- Chen PQ, Li GQ, Guo XB. The infectivity of gametocytes of Plasmodium falciparum from patients treated with artemisinin. Chinese Medical Journal. 1994;74((4)):209-10, 253-4. - PubMed
-
- Chen PQ, Li GQ, Guo XB, He KR, Fu YX, Fu LC, et al. The infectivity of gametocytes of Plasmodium falciparum from patients treated with artemisinin. Chinese Medical Journal. 1994;107((9)):709–11. - PubMed
-
- El-Sayed B, El-Zaki SE, Babiker H, Gadalla N, Ageep T, Mansour F, et al. A randomized open-label trial of artesunate-sulfadoxine-pyrimethamine with or without primaquine for elimination of sub-microscopic P. falciparum parasitaemia and gametocyte carriage in eastern Sudan. PLoS ONE. 2007;2((12)):e1311. - PMC - PubMed
References to studies excluded from this review
-
- Baird JK, Wiady I, Sutanihardja A, Suradi, Purnomo, Basri H, et al. Short report: therapeutic efficacy of chloroquine combined with primaquine against Plasmodium falciparum in northeastern Papua, Indonesia. 2002;66(6):659–60. American Journal of Tropical Medicine and Hygiene. - PubMed
-
- Barber MA, Komp WHW, Newman BM. The effect of small doses of plasmochin on the viability of gametocytes of malaria as measured by mosquito infection experiments. Public Health Reports. 1929;44((24)):1409–20.
-
- Barber MA, Rice JB, Brown JY. Malaria studies on the Firestone Rubber Plantation in Liberia, West Africa. American Journal of Hygiene. 1932;15((3)):601–33.
-
- Brueckner RP, Lasseter KC, Lin ET, Schuster BG. First-time-in-humans safety and pharmacokinetics of WR 238605, a new antimalarial. American Journal of Tropical Medicine & Hygiene. 1998;58((5)):645–9. - PubMed
-
- Bunnag D, Harinasuta T, Pinichpongse D, Suntharasamai P. Effect of primaquine on gametocytes of Plasmodium falciparum in Thailand. Lancet. 1980;2((8185)):91. - PubMed
References to studies awaiting assessment
-
- Chen L. Efficacy of artemether/primaquine against drug resistant P. falciparum. Journal of Applied Medicine. 1993;1((1)):31–3. [Chinese]
-
- Ishii A, Ohta N, Owhashi M, Kawabata M, Chung D, Bobogare A, et al. Trials of transmission blocking of P. falciparum with single dose primaquine in villages of Solomon Islands. MIM conference October 2009 MIM 16723361.
-
- Li J, et al. Artemether combined with primaquine for treatment of 50 Pf cases. Journal of Applied Medicine. 2006;22((19)):2299–300. [Chinese]
References to ongoing studies
-
- Primaquine's Gametocytocidal Efficacy in Malaria Asymptomatic Carriers Treated With Dihydroartemisinin-piperaquine in The Gambia. Ongoing study August 2013; December 2014 (final data collection date for primary outcome measure)
-
- Phase 2a Dose Escalation Study of the Efficacy, Safety, and Pharmacokinetics of Low Dose Primaquine for Gametocytocidal Activity Against P. Falciparum in Sub-Saharan Africa and South East Asia. Ongoing study September 2014 (final data collection date for primary outcome measure)
-
- Surveillance and Treatment With Dihydroartemisinin-piperaquine Plus Primaquine (MTC Belu)Sub-title: Impact of Mass Screening and Selective Treatment With Dihydroartemisinin-piperaquine Plus Primaquine on Malaria Transmission in High Endemic Area, Belu Regency, Nusa Tenggara Timur Province, Indonesia: a Randomized Cluster Trial. Ongoing study June 2013.
-
- Active Surveillance for P. falciparum Drug Resistance With Assessment of Transmission Blocking Activity of Single Dose Primaquine in Cambodia. Ongoing study December 2012; December 2014 (final data collection date for primary outcome measure)
-
- The Optimal Timing of Primaquine to Prevent Malaria Transmission After Artemisinin-Combination Therapy. Ongoing study May 2013; October 2013 (final data collection date for primary outcome measure)
Additional references
-
- Arnold J, Alving AS, Hockwald RS, Clayman CB, Dern RJ, Beutler E, et al. The antimalarial action of primaquine against the blood and tissue stages of falciparum malaria (Panama, P-F-6 strain) Journal of Laboratory and Clinical Medicine. 1955;46((3)):391–7. - PubMed
-
- Barnes, Ki, Little F, Mabuza A, Mngomezulu N, Govere J, Durrheim D, et al. Increased gametocytemia after treatment: an early parasitological indicator of emerging sulfadoxine-pyrimethamine resistance in falciparum malaria. Journal of Infectious Diseases. 2008;197((11)):1605–13. - PubMed
-
- Bhasin VK, Trager W. Chapter XI: Gametocytocidal effects in vitro of primaquine and related compounds on Plasmodium falciparum. In: Wernsdorfer WH, Trigg PI, editors. Primaquine: pharmacokinetics, metabolism, toxicity and activity. UNDP/World Bank/WHO; 1984.
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

