Large-Scale Interlaboratory Study to Develop, Analytically Validate and Apply Highly Multiplexed, Quantitative Peptide Assays to Measure Cancer-Relevant Proteins in Plasma
- PMID: 25693799
- PMCID: PMC4563721
- DOI: 10.1074/mcp.M114.047050
Large-Scale Interlaboratory Study to Develop, Analytically Validate and Apply Highly Multiplexed, Quantitative Peptide Assays to Measure Cancer-Relevant Proteins in Plasma
Abstract
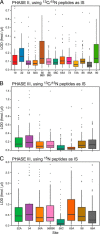
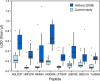
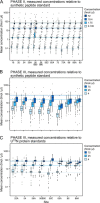
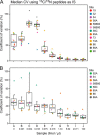
There is an increasing need in biology and clinical medicine to robustly and reliably measure tens to hundreds of peptides and proteins in clinical and biological samples with high sensitivity, specificity, reproducibility, and repeatability. Previously, we demonstrated that LC-MRM-MS with isotope dilution has suitable performance for quantitative measurements of small numbers of relatively abundant proteins in human plasma and that the resulting assays can be transferred across laboratories while maintaining high reproducibility and quantitative precision. Here, we significantly extend that earlier work, demonstrating that 11 laboratories using 14 LC-MS systems can develop, determine analytical figures of merit, and apply highly multiplexed MRM-MS assays targeting 125 peptides derived from 27 cancer-relevant proteins and seven control proteins to precisely and reproducibly measure the analytes in human plasma. To ensure consistent generation of high quality data, we incorporated a system suitability protocol (SSP) into our experimental design. The SSP enabled real-time monitoring of LC-MRM-MS performance during assay development and implementation, facilitating early detection and correction of chromatographic and instrumental problems. Low to subnanogram/ml sensitivity for proteins in plasma was achieved by one-step immunoaffinity depletion of 14 abundant plasma proteins prior to analysis. Median intra- and interlaboratory reproducibility was <20%, sufficient for most biological studies and candidate protein biomarker verification. Digestion recovery of peptides was assessed and quantitative accuracy improved using heavy-isotope-labeled versions of the proteins as internal standards. Using the highly multiplexed assay, participating laboratories were able to precisely and reproducibly determine the levels of a series of analytes in blinded samples used to simulate an interlaboratory clinical study of patient samples. Our study further establishes that LC-MRM-MS using stable isotope dilution, with appropriate attention to analytical validation and appropriate quality control measures, enables sensitive, specific, reproducible, and quantitative measurements of proteins and peptides in complex biological matrices such as plasma.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Picotti P., Aebersold R. (2012) Selected reaction monitoring-based proteomics: workflows, potential, pitfalls and future directions. Nature Meth. 9, 555–566 - PubMed
-
- Marx V. (2013) Targeted proteomics. Nature Meth. 10, 19–22 - PubMed
-
- Addona T. A., Abbatiello S. E., Schilling B., Skates S. J., Mani D. R., Bunk D. M., Spiegelman C. H., Zimmerman L. J., Ham A. J., Keshishian H., Hall S. C., Allen S., Blackman R. K., Borchers C. H., Buck C., Cardasis H. L., Cusack M. P., Dodder N. G., Gibson B. W., Held J. M., Hiltke T., Jackson A., Johansen E. B., Kinsinger C. R., Li J., Mesri M., Neubert T. A., Niles R. K., Pulsipher T. C., Ransohoff D., Rodriguez H., Rudnick P. A., Smith D., Tabb D. L., Tegeler T. J., Variyath A. M., Vega-Montoto L. J., Wahlander A., Waldemarson S., Wang M., Whiteaker J. R., Zhao L., Anderson N. L., Fisher S. J., Liebler D. C., Paulovich A. G., Regnier F. E., Tempst P., Carr S. A. (2009) Multi-site assessment of the precision and reproducibility of multiple reaction monitoring-based measurements of proteins in plasma. Nature Biotech. 27, 633–641 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U24 CA160034/CA/NCI NIH HHS/United States
- P30 CA016087/CA/NCI NIH HHS/United States
- U24 CA126485/CA/NCI NIH HHS/United States
- U24 CA159988/CA/NCI NIH HHS/United States
- U24CA159988/CA/NCI NIH HHS/United States
- R01 GM103551/GM/NIGMS NIH HHS/United States
- U24 126479/PHS HHS/United States
- U24 126480/PHS HHS/United States
- S10 RR027953/RR/NCRR NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U24 CA126476/CA/NCI NIH HHS/United States
- U24 126477/PHS HHS/United States
- U24CA160034/CA/NCI NIH HHS/United States
- S10 RR024604/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

