Diquat causes caspase-independent cell death in SH-SY5Y cells by production of ROS independently of mitochondria
- PMID: 25693864
- PMCID: PMC4572080
- DOI: 10.1007/s00204-015-1453-5
Diquat causes caspase-independent cell death in SH-SY5Y cells by production of ROS independently of mitochondria
Erratum in
-
Erratum to: Diquat causes caspase-independent cell death in SH-SY5Y cells by production of ROS independently of mitochondria.Arch Toxicol. 2015 Oct;89(10):1827. doi: 10.1007/s00204-015-1542-5. Arch Toxicol. 2015. PMID: 26084419 Free PMC article. No abstract available.
Abstract
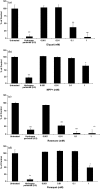
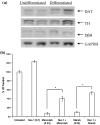
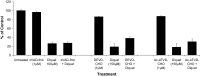
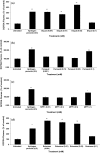
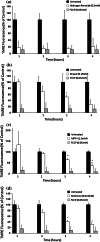
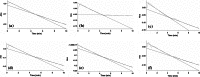
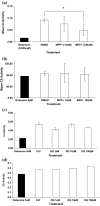
Evidence indicates that Parkinson's disease (PD), in addition to having a genetic aetiology, has an environmental component that contributes to disease onset and progression. The exact nature of any environmental agent contributing to PD is unknown in most cases. Given its similarity to paraquat, an agrochemical removed from registration in the EU for its suspected potential to cause PD, we have investigated the in vitro capacity of the related herbicide Diquat to cause PD-like cell death. Diquat showed greater toxicity towards SH-SY5Y neuroblastoma cells and human midbrain neural cells than paraquat and also MPTP, which was independent of dopamine transporter-mediated uptake. Diquat caused cell death independently of caspase activation, potentially via RIP1 kinase, with only a minor contribution from apoptosis, which was accompanied by enhanced reactive oxygen species production in the absence of major inhibition of complex I of the mitochondrial respiratory chain. No changes in α-synuclein expression were observed following 24-h or 4-week exposure. Diquat may, therefore, kill neural tissue by programmed necrosis rather than apoptosis, reflecting the pathological changes seen following high-level exposure, although its ability to promote PD is unclear.
Keywords: Apoptosis; Diquat; Mitochondria; Necrosis; Parkinson’s disease; Pesticide.
Figures



References
-
- Amstad PA, Yu G, Johnson GL, Lee BW, Dhawan S, Phelps DJ (2001) Detection of caspase activation in situ by fluorochrome-labeled caspase inhibitors. Biotechniques 31:608–610, 612, 614, passim - PubMed
-
- Barroso N, Campos Y, Huertas R, Esteban J, Molina JA, Alonso A, Gutierrez-Rivas E, Arenas J. Respiratory chain enzyme activities in lymphocytes from untreated patients with Parkinson disease. Clin Chem. 1993;39:667–669. - PubMed
-
- Barsoum MJ, Yuan H, Gerencser AA, Liot G, Kushnareva Y, Graber S, Kovacs I, Lee WD, Waggoner J, Cui J, White AD, Bossy B, Martinou JC, Youle RJ, Lipton SA, Ellisman MH, Perkins GA, Bossy-Wetzel E. Nitric oxide-induced mitochondrial fission is regulated by dynamin-related GTPases in neurons. EMBO J. 2006;25:3900–3911. doi: 10.1038/sj.emboj.7601253. - DOI - PMC - PubMed
-
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

