Peroxisomal Pex3 activates selective autophagy of peroxisomes via interaction with the pexophagy receptor Atg30
- PMID: 25694426
- PMCID: PMC4375511
- DOI: 10.1074/jbc.M114.619338
Peroxisomal Pex3 activates selective autophagy of peroxisomes via interaction with the pexophagy receptor Atg30
Abstract
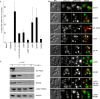
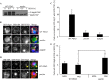
Pexophagy is a process that selectively degrades peroxisomes by autophagy. The Pichia pastoris pexophagy receptor Atg30 is recruited to peroxisomes under peroxisome proliferation conditions. During pexophagy, Atg30 undergoes phosphorylation, a prerequisite for its interactions with the autophagy scaffold protein Atg11 and the ubiquitin-like protein Atg8. Atg30 is subsequently shuttled to the vacuole along with the targeted peroxisome for degradation. Here, we defined the binding site for Atg30 on the peroxisomal membrane protein Pex3 and uncovered a role for Pex3 in the activation of Atg30 via phosphorylation and in the recruitment of Atg11 to the receptor protein complex. Pex3 is classically a docking protein for other proteins that affect peroxisome biogenesis, division, and segregation. We conclude that Pex3 has a role beyond simple docking of Atg30 and that its interaction with Atg30 regulates pexophagy in the yeast P. pastoris.
Keywords: Autophagy; Membrane Trafficking; Peroxisome; Pexophagy; Protein-Protein Interaction; Receptor Regulation; Signaling.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Tan C. C., Yu J. T., Tan M. S., Jiang T., Zhu X. C., Tan L. (2014) Autophagy in aging and neurodegenerative diseases: implications for pathogenesis and therapy. Neurobiol. Aging 35, 941–957 - PubMed
-
- Nakatogawa H., Suzuki K., Kamada Y., Ohsumi Y. (2009) Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat. Rev. Mol. Cell Biol. 10, 458–467 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

