bHLH proteins involved in Drosophila neurogenesis are mutually regulated at the level of stability
- PMID: 25694512
- PMCID: PMC4357701
- DOI: 10.1093/nar/gkv083
bHLH proteins involved in Drosophila neurogenesis are mutually regulated at the level of stability
Abstract
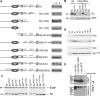
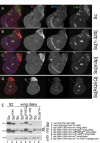
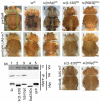
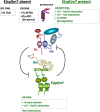
Proneural bHLH activators are expressed in all neuroectodermal regions prefiguring events of central and peripheral neurogenesis. Drosophila Sc is a prototypical proneural activator that heterodimerizes with the E-protein Daughterless (Da) and is antagonized by, among others, the E(spl) repressors. We determined parameters that regulate Sc stability in Drosophila S2 cells. We found that Sc is a very labile phosphoprotein and its turnover takes place via at least three proteasome-dependent mechanisms. (i) When Sc is in excess of Da, its degradation is promoted via its transactivation domain (TAD). (ii) In a DNA-bound Da/Sc heterodimer, Sc degradation is promoted via an SPTSS phosphorylation motif and the AD1 TAD of Da; Da is spared in the process. (iii) When E(spl)m7 is expressed, it complexes with Sc or Da/Sc and promotes their degradation in a manner that requires the corepressor Groucho and the Sc SPTSS motif. Da/Sc reciprocally promotes E(spl)m7 degradation. Since E(spl)m7 is a direct target of Notch, the mutual destabilization of Sc and E(spl) may contribute in part to the highly conserved anti-neural activity of Notch. Sc variants lacking the SPTSS motif are dramatically stabilized and are hyperactive in transgenic flies. Our results propose a novel mechanism of regulation of neurogenesis, involving the stability of key players in the process.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures




Similar articles
-
Essential roles of Da transactivation domains in neurogenesis and in E(spl)-mediated repression.Mol Cell Biol. 2012 Nov;32(22):4534-48. doi: 10.1128/MCB.00827-12. Epub 2012 Sep 4. Mol Cell Biol. 2012. PMID: 22949507 Free PMC article.
-
Role of the Sc C terminus in transcriptional activation and E(spl) repressor recruitment.J Biol Chem. 2005 Jan 14;280(2):1299-305. doi: 10.1074/jbc.M408949200. Epub 2004 Oct 26. J Biol Chem. 2005. PMID: 15507447
-
Two modes of recruitment of E(spl) repressors onto target genes.Development. 2003 Jan;130(2):259-70. doi: 10.1242/dev.00206. Development. 2003. PMID: 12466194
-
E(spl): genetic, developmental, and evolutionary aspects of a group of invertebrate Hes proteins with close ties to Notch signaling.Curr Top Dev Biol. 2014;110:217-62. doi: 10.1016/B978-0-12-405943-6.00006-3. Curr Top Dev Biol. 2014. PMID: 25248478 Review.
-
Vertebrate hairy and Enhancer of split related proteins: transcriptional repressors regulating cellular differentiation and embryonic patterning.Oncogene. 2001 Dec 20;20(58):8342-57. doi: 10.1038/sj.onc.1205094. Oncogene. 2001. PMID: 11840327 Review.
Cited by
-
ASC proneural factors are necessary for chromatin remodeling during neuroectodermal to neuroblast fate transition to ensure the timely initiation of the neural stem cell program.BMC Biol. 2022 May 13;20(1):107. doi: 10.1186/s12915-022-01300-8. BMC Biol. 2022. PMID: 35549704 Free PMC article.
-
Regulation of Notch output dynamics via specific E(spl)-HLH factors during bristle patterning in Drosophila.Nat Commun. 2019 Aug 2;10(1):3486. doi: 10.1038/s41467-019-11477-2. Nat Commun. 2019. PMID: 31375669 Free PMC article.
-
The Id protein Extramacrochaetae restrains the E protein Daughterless to regulate Notch, Rap1, and Sevenless within the R7 equivalence group of the Drosophila eye.Biol Open. 2024 Aug 15;13(8):bio060124. doi: 10.1242/bio.060124. Epub 2024 Aug 20. Biol Open. 2024. PMID: 39041866 Free PMC article.
-
Regulation of the Drosophila ID protein Extra macrochaetae by proneural dimerization partners.Elife. 2018 Apr 24;7:e33967. doi: 10.7554/eLife.33967. Elife. 2018. PMID: 29687780 Free PMC article.
-
All in the family: proneural bHLH genes and neuronal diversity.Development. 2018 May 2;145(9):dev159426. doi: 10.1242/dev.159426. Development. 2018. PMID: 29720483 Free PMC article. Review.
References
-
- Villares R., Cabrera C.V. The achaete-scute gene complex of D. melanogaster: conserved domains in a subset of genes required for neurogenesis and their homology to myc. Cell. 1987;50:415–424. - PubMed
-
- Guillemot F., Lo L.C., Johnson J.E., Auerbach A., Anderson D.J., Joyner A.L. Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell. 1993;75:463–476. - PubMed
-
- Bertrand N., Castro D.S., Guillemot F. Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 2002;3:517–530. - PubMed
-
- Campuzano S., Balcells L., Villares R., Carramolino L., Garcia-Alonso L., Modolell J. Excess function hairy-wing mutations caused by gypsy and copia insertions within structural genes of the achaete-scute locus of Drosophila. Cell. 1986;44:303–312. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

