A novel endonuclease that may be responsible for damaged DNA base repair in Pyrococcus furiosus
- PMID: 25694513
- PMCID: PMC4357722
- DOI: 10.1093/nar/gkv121
A novel endonuclease that may be responsible for damaged DNA base repair in Pyrococcus furiosus
Abstract
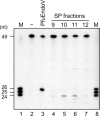
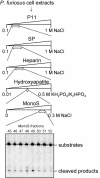
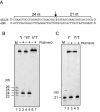
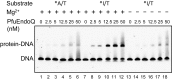
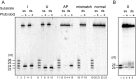
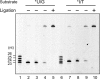
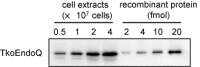
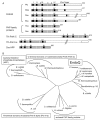
DNA is constantly damaged by endogenous and environmental influences. Deaminated adenine (hypoxanthine) tends to pair with cytosine and leads to the A:T→G:C transition mutation during DNA replication. Endonuclease V (EndoV) hydrolyzes the second phosphodiester bond 3' from deoxyinosine in the DNA strand, and was considered to be responsible for hypoxanthine excision repair. However, the downstream pathway after EndoV cleavage remained unclear. The activity to cleave the phosphodiester bond 5' from deoxyinosine was detected in a Pyrococcus furiosus cell extract. The protein encoded by PF1551, obtained from the mass spectrometry analysis of the purified fraction, exhibited the corresponding cleavage activity. A putative homolog from Thermococcus kodakarensis (TK0887) showed the same activity. Further biochemical analyses revealed that the purified PF1551 and TK0887 proteins recognize uracil, xanthine and the AP site, in addition to hypoxanthine. We named this endonuclease Endonuclease Q (EndoQ), as it may be involved in damaged base repair in the Thermococcals of Archaea.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures

Similar articles
-
Molecular Basis of Substrate Recognition of Endonuclease Q from the Euryarchaeon Pyrococcus furiosus.J Bacteriol. 2020 Jan 2;202(2):e00542-19. doi: 10.1128/JB.00542-19. Print 2020 Jan 2. J Bacteriol. 2020. PMID: 31685534 Free PMC article.
-
Structural basis for recognition of distinct deaminated DNA lesions by endonuclease Q.Proc Natl Acad Sci U S A. 2021 Mar 9;118(10):e2021120118. doi: 10.1073/pnas.2021120118. Proc Natl Acad Sci U S A. 2021. PMID: 33658373 Free PMC article.
-
EndoQ and EndoV work individually for damaged DNA base repair in Pyrococcus furiosus.Biochimie. 2015 Nov;118:264-9. doi: 10.1016/j.biochi.2015.06.015. Epub 2015 Jun 24. Biochimie. 2015. PMID: 26116888
-
Studies on the base excision repair (BER) complex in Pyrococcus furiosus.Biochem Soc Trans. 2009 Feb;37(Pt 1):79-82. doi: 10.1042/BST0370079. Biochem Soc Trans. 2009. PMID: 19143606 Review.
-
Structural basis for incision at deaminated adenines in DNA and RNA by endonuclease V.Prog Biophys Mol Biol. 2015 Mar;117(2-3):134-142. doi: 10.1016/j.pbiomolbio.2015.03.005. Epub 2015 Mar 28. Prog Biophys Mol Biol. 2015. PMID: 25824682 Review.
Cited by
-
Molecular Basis of Substrate Recognition of Endonuclease Q from the Euryarchaeon Pyrococcus furiosus.J Bacteriol. 2020 Jan 2;202(2):e00542-19. doi: 10.1128/JB.00542-19. Print 2020 Jan 2. J Bacteriol. 2020. PMID: 31685534 Free PMC article.
-
Repair of Hypoxanthine in DNA Revealed by DNA Glycosylases and Endonucleases From Hyperthermophilic Archaea.Front Microbiol. 2021 Aug 31;12:736915. doi: 10.3389/fmicb.2021.736915. eCollection 2021. Front Microbiol. 2021. PMID: 34531846 Free PMC article. Review.
-
Structural basis for recognition of distinct deaminated DNA lesions by endonuclease Q.Proc Natl Acad Sci U S A. 2021 Mar 9;118(10):e2021120118. doi: 10.1073/pnas.2021120118. Proc Natl Acad Sci U S A. 2021. PMID: 33658373 Free PMC article.
-
Evolutionary Origins of DNA Repair Pathways: Role of Oxygen Catastrophe in the Emergence of DNA Glycosylases.Cells. 2021 Jun 24;10(7):1591. doi: 10.3390/cells10071591. Cells. 2021. PMID: 34202661 Free PMC article. Review.
-
Haloferax volcanii-a model archaeon for studying DNA replication and repair.Open Biol. 2020 Dec;10(12):200293. doi: 10.1098/rsob.200293. Epub 2020 Dec 2. Open Biol. 2020. PMID: 33259746 Free PMC article. Review.
References
-
- Parsons J.L., Dianov G.L. Co-ordination of base excision repair and genome stability. DNA Repair. 2013;12:326–333. - PubMed
-
- Kuper J., Kisker C. Damage recognition in nucleotide excision DNA repair. Curr. Opin. Struct. Biol. 2012;22:88–93. - PubMed
-
- Naegeli H., Sugasawa K. The xeroderma pigmentosum pathway: decision tree analysis of DNA quality. DNA Repair. 2011;10:673–683. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

