Comparative evaluation of three commercial quantitative cytomegalovirus standards by use of digital and real-time PCR
- PMID: 25694529
- PMCID: PMC4400765
- DOI: 10.1128/JCM.03375-14
Comparative evaluation of three commercial quantitative cytomegalovirus standards by use of digital and real-time PCR
Abstract
The recent development of the 1st WHO International Standard for human cytomegalovirus (CMV) and the introduction of commercially produced secondary standards have raised hopes of improved agreement among laboratories performing quantitative PCR for CMV. However, data to evaluate the trueness and uniformity of secondary standards and the consistency of results achieved when these materials are run on various assays are lacking. Three concentrations of each of the three commercially prepared secondary CMV standards were tested in quadruplicate by three real-time and two digital PCR methods. The mean results were compared in a pairwise fashion with nominal values provided by each manufacturer. The agreement of results among all methods for each sample and for like concentrations of each standard was also assessed. The relationship between the nominal values of standards and the measured values varied, depending upon the assay used and the manufacturer of the standards, with the degree of bias ranging from +0.6 to -1.0 log10 IU/ml. The mean digital PCR result differed significantly among the secondary standards, as did the results of the real-time PCRs, particularly when plotted against nominal log10 IU values. Commercially available quantitative secondary CMV standards produce variable results when tested by different real-time and digital PCR assays, with various magnitudes of bias compared to nominal values. These findings suggest that the use of such materials may not achieve the intended uniformity among laboratories measuring CMV viral load, as envisioned by adaptation of the WHO standard.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

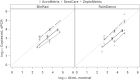

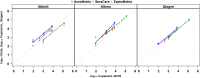
References
-
- Gerna G, Campanini G, Rognoni V, Marchi A, Rovida F, Piralla A, Percivalle E. 2008. Correlation of viral load as determined by real-time RT-PCR and clinical characteristics of respiratory syncytial virus lower respiratory tract infections in early infancy. J Clin Virol 41:45–48. doi:10.1016/j.jcv.2007.10.018. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

