Identification and expression of Babesia ovis secreted antigen 1 and evaluation of its diagnostic potential in an enzyme-linked immunosorbent assay
- PMID: 25694531
- PMCID: PMC4400748
- DOI: 10.1128/JCM.03219-14
Identification and expression of Babesia ovis secreted antigen 1 and evaluation of its diagnostic potential in an enzyme-linked immunosorbent assay
Abstract
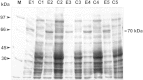
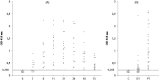
In order to identify immunoreactive proteins that are usable for the immunological diagnosis of Babesia ovis infections, a phage lambda cDNA expression library was constructed and screened using parasite-specific immune serum. Immunoscreening resulted in the identification of a full-length cDNA clone encoding a secreted protein designated Babesia ovis secreted antigen 1 (BoSA1). The full-length BoSA1 cDNA contained a 1,137-bp open reading frame that encoded a protein of 378 amino acids, with a signal peptide and 2 internal repeat domains. The theoretical molecular mass of the mature protein was 42.5 kDa. Recombinant BoSA1 (rBoSA1) protein was expressed in Escherichia coli strain DH5α cells as a glutathione S-transferase (GST) fusion protein and was purified by affinity chromatography. Purified rBoSA1 was tested for reactivity with sera from animals experimentally or naturally infected with B. ovis, in an indirect enzyme-linked immunosorbent assay (ELISA). The results showed that specific antibodies against rBoSA1 were detectable on days 7 and 8 of the experimental infection and were maintained during the sampling period. Additionally, 38 field sera taken from sheep naturally infected with B. ovis gave strong positive reactions in the ELISA between day 20 and day 30 of treatment. As a result, the identified recombinant BoSA1 protein seems to be a promising diagnostic antigen that is usable for the development of serological assays for the diagnosis of ovine babesiosis. This is the first report on the molecular cloning, expression, and potential use of a recombinant antigen for the diagnosis of ovine babesiosis.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures



Similar articles
-
Babesia ovis secreted antigen-1 is a diagnostic marker during the active Babesia ovis infections in sheep.Front Cell Infect Microbiol. 2023 Aug 16;13:1238369. doi: 10.3389/fcimb.2023.1238369. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37662014 Free PMC article.
-
A new immunoreactive recombinant protein designated as rBoSA2 from Babesia ovis: Its molecular characterization, subcellular localization and antibody recognition by infected sheep.Vet Parasitol. 2015 Nov 30;214(1-2):213-8. doi: 10.1016/j.vetpar.2015.09.022. Epub 2015 Sep 25. Vet Parasitol. 2015. PMID: 26428018
-
Expression of sheep pathogen Babesia sp. Xinjiang rhoptry-associated protein 1 and evaluation of its diagnostic potential by enzyme-linked immunosorbent assay.Parasitology. 2016 Dec;143(14):1990-1999. doi: 10.1017/S0031182016001293. Epub 2016 Oct 17. Parasitology. 2016. PMID: 27748232
-
Serological diagnostic tools for the major tick-borne protozoan diseases of livestock.Parassitologia. 2007 May;49 Suppl 1:53-62. Parassitologia. 2007. PMID: 17691608 Review.
-
Recently developed methods for the detection of babesial infections.Trans R Soc Trop Med Hyg. 1989;83 Suppl:21-3. doi: 10.1016/0035-9203(89)90598-1. Trans R Soc Trop Med Hyg. 1989. PMID: 2696156 Review.
Cited by
-
Role of Rhipicephalus bursa larvae in transstadial transmission and endemicity of Babesia ovis in chronically infected sheep.Front Cell Infect Microbiol. 2024 Jul 26;14:1428719. doi: 10.3389/fcimb.2024.1428719. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39131920 Free PMC article.
-
Sheep Displayed No Clinical and Parasitological Signs upon Experimental Infection with Babesia aktasi.Vet Sci. 2024 Aug 8;11(8):359. doi: 10.3390/vetsci11080359. Vet Sci. 2024. PMID: 39195813 Free PMC article.
-
Primary Tick-Borne Protozoan and Rickettsial Infections of Animals in Turkey.Pathogens. 2021 Feb 19;10(2):231. doi: 10.3390/pathogens10020231. Pathogens. 2021. PMID: 33669573 Free PMC article. Review.
-
Whole genome sequence and diversity in multigene families of Babesia ovis.Front Cell Infect Microbiol. 2023 Aug 1;13:1194608. doi: 10.3389/fcimb.2023.1194608. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37662008 Free PMC article.
-
Babesia ovis secreted antigen-1 is a diagnostic marker during the active Babesia ovis infections in sheep.Front Cell Infect Microbiol. 2023 Aug 16;13:1238369. doi: 10.3389/fcimb.2023.1238369. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37662014 Free PMC article.
References
-
- Kuttler KL. 1988. World-wide impact of babesiosis, p 1–22. In Ristic M. (ed), Babesiosis of domestic animals and man. CRC Press, Boca Raton, FL.
-
- Razmi GR, Naghibi A, Aslani MR, Dastjerdi K, Hossieni H. 2003. An epidemiological study on Babesia infection in small ruminants in Mashhad suburb, Khorasan Province, Iran. Small Rumin Res 50:39–44. doi:10.1016/S0921-4488(03)00107-X. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

