Murine coronavirus ubiquitin-like domain is important for papain-like protease stability and viral pathogenesis
- PMID: 25694594
- PMCID: PMC4403493
- DOI: 10.1128/JVI.00338-15
Murine coronavirus ubiquitin-like domain is important for papain-like protease stability and viral pathogenesis
Abstract
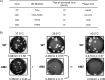
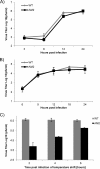
Ubiquitin-like domains (Ubls) now are recognized as common elements adjacent to viral and cellular proteases; however, their function is unclear. Structural studies of the papain-like protease (PLP) domains of coronaviruses (CoVs) revealed an adjacent Ubl domain in severe acute respiratory syndrome CoV, Middle East respiratory syndrome CoV, and the murine CoV, mouse hepatitis virus (MHV). Here, we tested the effect of altering the Ubl adjacent to PLP2 of MHV on enzyme activity, viral replication, and pathogenesis. Using deletion and substitution approaches, we identified sites within the Ubl domain, residues 785 to 787 of nonstructural protein 3, which negatively affect protease activity, and valine residues 785 and 787, which negatively affect deubiquitinating activity. Using reverse genetics, we engineered Ubl mutant viruses and found that AM2 (V787S) and AM3 (V785S) viruses replicate efficiently at 37°C but generate smaller plaques than wild-type (WT) virus, and AM2 is defective for replication at higher temperatures. To evaluate the effect of the mutation on protease activity, we purified WT and Ubl mutant PLP2 and found that the proteases exhibit similar specific activities at 25°C. However, the thermal stability of the Ubl mutant PLP2 was significantly reduced at 30°C, thereby reducing the total enzymatic activity. To determine if the destabilizing mutation affects viral pathogenesis, we infected C57BL/6 mice with WT or AM2 virus and found that the mutant virus is highly attenuated, yet it replicates sufficiently to elicit protective immunity. These studies revealed that modulating the Ubl domain adjacent to the PLP reduces protease stability and viral pathogenesis, revealing a novel approach to coronavirus attenuation.
Importance: Introducing mutations into a protein or virus can have either direct or indirect effects on function. We asked if changes in the Ubl domain, a conserved domain adjacent to the coronavirus papain-like protease, altered the viral protease activity or affected viral replication or pathogenesis. Our studies using purified wild-type and Ubl mutant proteases revealed that mutations in the viral Ubl domain destabilize and inactivate the adjacent viral protease. Furthermore, we show that a CoV encoding the mutant Ubl domain is unable to replicate at high temperature or cause lethal disease in mice. Our results identify the coronavirus Ubl domain as a novel modulator of viral protease stability and reveal manipulating the Ubl domain as a new approach for attenuating coronavirus replication and pathogenesis.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures




References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

