Oncogenic human T-cell lymphotropic virus type 1 tax suppression of primary innate immune signaling pathways
- PMID: 25694597
- PMCID: PMC4403453
- DOI: 10.1128/JVI.02493-14
Oncogenic human T-cell lymphotropic virus type 1 tax suppression of primary innate immune signaling pathways
Abstract
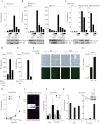
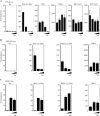
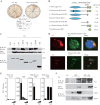
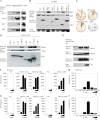
Human T-cell lymphotropic virus type I (HTLV-1) is an oncogenic retrovirus considered to be the etiological agent of adult T-cell leukemia (ATL). The viral transactivator Tax is regarded as the oncoprotein responsible for contributing toward the transformation process. Here, we demonstrate that Tax potently inhibits the activity of DEx(D/H) box helicases RIG-I and MDA5 as well as Toll-dependent TIR-domain-containing adapter-inducing interferon-β (TRIF), which function as cellular sensors or mediators of viral RNA and facilitate innate immune responses, including the production of type I IFN. Tax manifested this function by binding to the RIP homotypic interaction motif (RHIM) domains of TRIF and RIP1 to disrupt interferon regulatory factor 7 (IRF7) activity, a critical type I IFN transcription factor. These data provide further mechanistic insight into HTLV-1-mediated subversion of cellular host defense responses, which may help explain HTLV-1-related pathogenesis and oncogenesis.
Importance: It is predicted that up to 15% of all human cancers may involve virus infection. For example, human T-cell lymphotropic virus type 1 (HTLV-1) has been reported to infect up to 25 million people worldwide and is the causative agent of adult T-cell leukemia (ATL). We show here that HTLV-1 may be able to successfully infect the T cells and remain latent due to the virally encoded product Tax inhibiting a key host defense pathway. Understanding the mechanisms by which Tax subverts the immune system may lead to the development of a therapeutic treatment for HTLV-1-mediated disease.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures


Similar articles
-
Suppression of Type I Interferon Production by Human T-Cell Leukemia Virus Type 1 Oncoprotein Tax through Inhibition of IRF3 Phosphorylation.J Virol. 2016 Mar 28;90(8):3902-3912. doi: 10.1128/JVI.00129-16. Print 2016 Apr. J Virol. 2016. PMID: 26819312 Free PMC article.
-
The Major Histocompatibility Complex Class II Transactivator CIITA Inhibits the Persistent Activation of NF-κB by the Human T Cell Lymphotropic Virus Type 1 Tax-1 Oncoprotein.J Virol. 2016 Jan 20;90(7):3708-21. doi: 10.1128/JVI.03000-15. J Virol. 2016. PMID: 26792751 Free PMC article.
-
Feedback Loop Regulation between Pim Kinases and Tax Keeps Human T-Cell Leukemia Virus Type 1 Viral Replication in Check.J Virol. 2022 Feb 9;96(3):e0196021. doi: 10.1128/JVI.01960-21. Epub 2021 Nov 24. J Virol. 2022. PMID: 34818069 Free PMC article.
-
Role of Tax protein in human T-cell leukemia virus type-I leukemogenicity.Retrovirology. 2004 Aug 13;1:20. doi: 10.1186/1742-4690-1-20. Retrovirology. 2004. PMID: 15310405 Free PMC article. Review.
-
HTLV-1 Tax: Linking transformation, DNA damage and apoptotic T-cell death.Chem Biol Interact. 2010 Nov 5;188(2):359-65. doi: 10.1016/j.cbi.2010.06.005. Epub 2010 Jun 15. Chem Biol Interact. 2010. PMID: 20558150 Review.
Cited by
-
Sensing of cell-associated HTLV by plasmacytoid dendritic cells is regulated by dense β-galactoside glycosylation.PLoS Pathog. 2019 Feb 28;15(2):e1007589. doi: 10.1371/journal.ppat.1007589. eCollection 2019 Feb. PLoS Pathog. 2019. PMID: 30818370 Free PMC article.
-
Epitope-based universal vaccine for Human T-lymphotropic virus-1 (HTLV-1).PLoS One. 2021 Apr 2;16(4):e0248001. doi: 10.1371/journal.pone.0248001. eCollection 2021. PLoS One. 2021. PMID: 33798232 Free PMC article.
-
Virus-Induced Tumorigenesis and IFN System.Biology (Basel). 2021 Oct 1;10(10):994. doi: 10.3390/biology10100994. Biology (Basel). 2021. PMID: 34681093 Free PMC article. Review.
-
Positive and Negative Regulation of Type I Interferons by the Human T Cell Leukemia Virus Antisense Protein HBZ.J Virol. 2017 Sep 27;91(20):e00853-17. doi: 10.1128/JVI.00853-17. Print 2017 Oct 15. J Virol. 2017. PMID: 28768861 Free PMC article.
-
Human T-cell leukaemia virus type 1: parasitism and pathogenesis.Philos Trans R Soc Lond B Biol Sci. 2017 Oct 19;372(1732):20160272. doi: 10.1098/rstb.2016.0272. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28893939 Free PMC article. Review.
References
-
- Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. 2002. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol 169:6668–6672. doi:10.4049/jimmunol.169.12.6668. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

