CCCTC-binding factor recruitment to the early region of the human papillomavirus 18 genome regulates viral oncogene expression
- PMID: 25694598
- PMCID: PMC4403478
- DOI: 10.1128/JVI.00097-15
CCCTC-binding factor recruitment to the early region of the human papillomavirus 18 genome regulates viral oncogene expression
Abstract
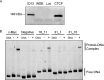
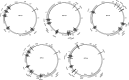
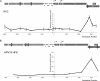
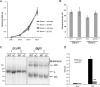
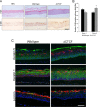
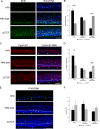
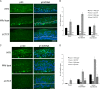
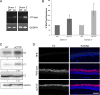
Host cell differentiation-dependent regulation of human papillomavirus (HPV) gene expression is required for productive infection. The host cell CCCTC-binding factor (CTCF) functions in genome-wide chromatin organization and gene regulation. We have identified a conserved CTCF binding site in the E2 open reading frame of high-risk HPV types. Using organotypic raft cultures of primary human keratinocytes containing high-risk HPV18 genomes, we show that CTCF recruitment to this conserved site regulates viral gene expression in differentiating epithelia. Mutation of the CTCF binding site increases the expression of the viral oncoproteins E6 and E7 and promotes host cell proliferation. Loss of CTCF binding results in a reduction of a specific alternatively spliced transcript expressed from the early gene region concomitant with an increase in the abundance of unspliced early transcripts. We conclude that high-risk HPV types have evolved to recruit CTCF to the early gene region to control the balance and complexity of splicing events that regulate viral oncoprotein expression.
Importance: The establishment and maintenance of HPV infection in undifferentiated basal cells of the squamous epithelia requires the activation of a subset of viral genes, termed early genes. The differentiation of infected cells initiates the expression of the late viral transcripts, allowing completion of the virus life cycle. This tightly controlled balance of differentiation-dependent viral gene expression allows the virus to stimulate cellular proliferation to support viral genome replication with minimal activation of the host immune response, promoting virus productivity. Alternative splicing of viral mRNAs further increases the complexity of viral gene expression. In this study, we show that the essential host cell protein CTCF, which functions in genome-wide chromatin organization and gene regulation, is recruited to the HPV genome and plays an essential role in the regulation of early viral gene expression and transcript processing. These data highlight a novel virus-host interaction important for HPV pathogenicity.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

Comment in
-
Long-distance communication: Looping of human papillomavirus genomes regulates expression of viral oncogenes.PLoS Biol. 2018 Nov 27;16(11):e3000062. doi: 10.1371/journal.pbio.3000062. eCollection 2018 Nov. PLoS Biol. 2018. PMID: 30481166 Free PMC article.
Similar articles
-
The chromatin insulator CTCF regulates HPV18 transcript splicing and differentiation-dependent late gene expression.PLoS Pathog. 2021 Nov 4;17(11):e1010032. doi: 10.1371/journal.ppat.1010032. eCollection 2021 Nov. PLoS Pathog. 2021. PMID: 34735550 Free PMC article.
-
Serine/Arginine-Rich Splicing Factor 3 and Heterogeneous Nuclear Ribonucleoprotein A1 Regulate Alternative RNA Splicing and Gene Expression of Human Papillomavirus 18 through Two Functionally Distinguishable cis Elements.J Virol. 2016 Sep 29;90(20):9138-52. doi: 10.1128/JVI.00965-16. Print 2016 Oct 15. J Virol. 2016. PMID: 27489271 Free PMC article.
-
Human papillomaviruses activate and recruit SMC1 cohesin proteins for the differentiation-dependent life cycle through association with CTCF insulators.PLoS Pathog. 2015 Apr 13;11(4):e1004763. doi: 10.1371/journal.ppat.1004763. eCollection 2015 Apr. PLoS Pathog. 2015. PMID: 25875106 Free PMC article.
-
Targeting CTCF to Control Virus Gene Expression: A Common Theme amongst Diverse DNA Viruses.Viruses. 2015 Jul 6;7(7):3574-85. doi: 10.3390/v7072791. Viruses. 2015. PMID: 26154016 Free PMC article. Review.
-
[Possible role of transcription factor AP1 in the tissue-specific regulation of human papillomavirus].Rev Invest Clin. 2002 May-Jun;54(3):231-42. Rev Invest Clin. 2002. PMID: 12183893 Review. Spanish.
Cited by
-
The SMC5/6 Complex Represses the Replicative Program of High-Risk Human Papillomavirus Type 31.Pathogens. 2020 Sep 25;9(10):786. doi: 10.3390/pathogens9100786. Pathogens. 2020. PMID: 32992873 Free PMC article.
-
RNA Binding Proteins that Control Human Papillomavirus Gene Expression.Biomolecules. 2015 May 5;5(2):758-74. doi: 10.3390/biom5020758. Biomolecules. 2015. PMID: 25950509 Free PMC article. Review.
-
The Role of E6 Spliced Isoforms (E6*) in Human Papillomavirus-Induced Carcinogenesis.Viruses. 2018 Jan 18;10(1):45. doi: 10.3390/v10010045. Viruses. 2018. PMID: 29346309 Free PMC article. Review.
-
Human papillomavirus integration transforms chromatin to drive oncogenesis.Genome Biol. 2023 Jun 27;24(1):142. doi: 10.1186/s13059-023-02926-9. Genome Biol. 2023. PMID: 37365652 Free PMC article.
-
Disruption of CTCF-YY1-dependent looping of the human papillomavirus genome activates differentiation-induced viral oncogene transcription.PLoS Biol. 2018 Oct 25;16(10):e2005752. doi: 10.1371/journal.pbio.2005752. eCollection 2018 Oct. PLoS Biol. 2018. PMID: 30359362 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

